Amantadine treatment is associated with improved consciousness in patients with non-traumatic brain injury
- PMID: 35086939
- PMCID: PMC9148986
- DOI: 10.1136/jnnp-2021-327408
Amantadine treatment is associated with improved consciousness in patients with non-traumatic brain injury
Abstract
Objective: This study determined the effect of amantadine treatment on consciousness in patients with non-traumatic brain injury.
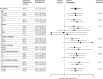
Methods: We pooled individual patient data of five single-centre observational studies to determine the effect of amantadine treatment among patients with ischaemic stroke, intracerebral haemorrhage, subarachnoid haemorrhage, community-acquired bacterial meningitis and status epilepticus, admitted between January 2012 and December 2015 and ventilated ≥7 days. Patient selection and multivariable regression modelling were used to adjust for differences in intergroup comparison and for parameters associated with consciousness. Improvement of consciousness 5 days after treatment initiation was defined as primary outcome. Secondary outcomes included Glasgow Coma Scale (GCS) at day 5 and GCS at day 10, rate of ICU delirium, epileptic seizures and all-cause mortality at 90 days.
Results: Overall, 84 of 294 (28.6%) eligible patients received amantadine. Amantadine treatment was associated with improvement of consciousness at day 5 (amantadine: 86.9% vs control: 54.0%; absolute difference: 32.9 (20.0-44.2); adjusted OR (aOR): 5.71 (2.50-13.05), p<0.001). Secondary outcomes showed differences in GCS 5 days (9 (8-11) vs 6 (3-9), p<0.001) and GCS 10 days (10(8-11) vs 9(6-11),p=0.003) after treatment initiation. There were no significant differences regarding all-cause mortality (aOR: 0.89 (0.44-1.82), p=0.758) and ICU delirium (aOR: 1.39 (0.58-3.31), p=0.462). Rate of epileptic seizures after initiation of amantadine treatment was numerically higher in the amantadine group (amantadine: 10.7% vs control: 3.0%; absolute difference: 7.7 (0.3-16.4); aOR: 3.68 (0.86-15.71), p=0.079).
Conclusions: Amantadine treatment is associated with improved consciousness among patients with different types of non-traumatic brain injury in this observational cohort analysis. Epileptic seizures should be considered as potential side effects and randomised controlled trials are needed to confirm these findings.
Keywords: acquired brain injury; consciousness; epilepsy; stroke; subarachnoid haemorrhage.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JBK reports personal fees from Bayer, personal fees from Bristol-Myers Squibb & Pfizer, personal fees from Sanofi, and personal fees from Boehringer Ingelheim outside the submitted work. BK reports personal fees from Bayer, personal fees from Bristol-Myers Squibb & Pfizer and personal fees from Medtronic outside the submitted work. HBH reports personal fees from Boehringer Ingelheim, personal fees from Bayer AG, personal fees from Daiichi Sankyo, grants and personal fees from Novartis, grants and personal fees from Portola Pharmaceuticals, grants and personal fees from Union Chimique Belge Pharma, and grants and personal fees from Medtronic outside the submitted work. MIS reports grants from IZKF and Marohn Foundation.
Figures


References
-
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators . Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 2019;18:56–87. 10.1016/S1474-4422(18)30415-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
