Structural biology of SARS-CoV-2: open the door for novel therapies
- PMID: 35087058
- PMCID: PMC8793099
- DOI: 10.1038/s41392-022-00884-5
Structural biology of SARS-CoV-2: open the door for novel therapies
Abstract
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) is the causative agent of the pandemic disease COVID-19, which is so far without efficacious treatment. The discovery of therapy reagents for treating COVID-19 are urgently needed, and the structures of the potential drug-target proteins in the viral life cycle are particularly important. SARS-CoV-2, a member of the Orthocoronavirinae subfamily containing the largest RNA genome, encodes 29 proteins including nonstructural, structural and accessory proteins which are involved in viral adsorption, entry and uncoating, nucleic acid replication and transcription, assembly and release, etc. These proteins individually act as a partner of the replication machinery or involved in forming the complexes with host cellular factors to participate in the essential physiological activities. This review summarizes the representative structures and typically potential therapy agents that target SARS-CoV-2 or some critical proteins for viral pathogenesis, providing insights into the mechanisms underlying viral infection, prevention of infection, and treatment. Indeed, these studies open the door for COVID therapies, leading to ways to prevent and treat COVID-19, especially, treatment of the disease caused by the viral variants are imperative.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
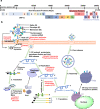



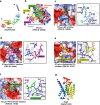
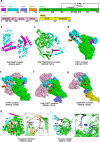
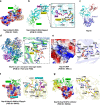

References
Publication types
MeSH terms
Substances
Grants and funding
- 31870836/National Natural Science Foundation of China (National Science Foundation of China)
- 31870836/National Natural Science Foundation of China (National Science Foundation of China)
- 31870836/National Natural Science Foundation of China (National Science Foundation of China)
- 31870836/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Miscellaneous

