CD97 is associated with mitogenic pathway activation, metabolic reprogramming, and immune microenvironment changes in glioblastoma
- PMID: 35087132
- PMCID: PMC8795421
- DOI: 10.1038/s41598-022-05259-y
CD97 is associated with mitogenic pathway activation, metabolic reprogramming, and immune microenvironment changes in glioblastoma
Abstract
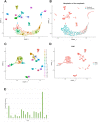
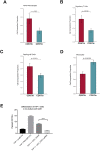
Glioblastoma (GBM) is the most common primary brain tumor with a median survival under two years. Using in silico and in vitro techniques, we demonstrate heterogeneous expression of CD97, a leukocyte adhesion marker, in human GBM. Beyond its previous demonstrated role in tumor invasion, we show that CD97 is also associated with upregulation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) and phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) pathways in GBM. While CD97 knockout decreased Akt activation, CD97 targeting did not alter MAPK/Erk activation, did not slow GBM cell proliferation in culture, and increased levels of glycolytic and oxidative phosphorylation metabolites. Treatment with a soluble CD97 inhibitor did not alter activation of the MAPK/Erk and PI3K/Akt pathways. Tumors with high CD97 expression were associated with immune microenvironment changes including increased naïve macrophages, regulatory T cells, and resting natural killer (NK) cells. These data suggest that, while CD97 expression is associated with conflicting effects on tumor cell proliferative and metabolic pathways that overall do not affect tumor cell proliferation, CD97 exerts pro-tumoral effects on the tumor immune microenvironment, which along with the pro-invasive effects of CD97 we previously demonstrated, provides impetus to continue exploring CD97 as a therapeutic target in GBM.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

