Synthesis and in vitro assay of hydroxyxanthones as antioxidant and anticancer agents
- PMID: 35087149
- PMCID: PMC8795354
- DOI: 10.1038/s41598-022-05573-5
Synthesis and in vitro assay of hydroxyxanthones as antioxidant and anticancer agents
Abstract
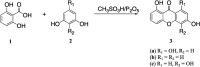
In the present work, three hydroxyxanthones were synthesized in 11.15-33.42% yield from 2,6-dihydroxybenzoic acid as the starting material. The chemical structures of prepared hydroxyxanthones have been elucidated by using spectroscopic techniques. Afterward, the hydroxyxanthones were evaluated as antioxidant agents through radical scavenging assay; and anticancer agents through in vitro assays against WiDr, MCF-7, and HeLa cancer cell lines. Hydroxyxanthone 3b was categorized as a strong antioxidant agent (IC50 = 349 ± 68 µM), while the other compounds were categorized as moderate antioxidant agents (IC50 > 500 µM). On the other hand, hydroxyxanthone 3a exhibited the highest anticancer activity (IC50 = 184 ± 15 µM) and the highest selectivity (SI = 18.42) against MCF-7 cancer cells. From the molecular docking study, it was found that hydroxyxanthone 3a interacted with the active sites of Topoisomerase II protein through Hydrogen bonding with DG13 and π-π stacking interactions with DA12 and DC8. These findings revealed that hydroxyxanthones are potential candidates to be developed as antioxidant and anticancer agents in the future.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous