Cerebellar Coordination of Neuronal Communication in Cerebral Cortex
- PMID: 35087384
- PMCID: PMC8787113
- DOI: 10.3389/fnsys.2021.781527
Cerebellar Coordination of Neuronal Communication in Cerebral Cortex
Abstract
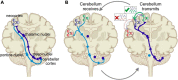
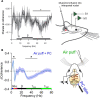
Cognitive processes involve precisely coordinated neuronal communications between multiple cerebral cortical structures in a task specific manner. Rich new evidence now implicates the cerebellum in cognitive functions. There is general agreement that cerebellar cognitive function involves interactions between the cerebellum and cerebral cortical association areas. Traditional views assume reciprocal interactions between one cerebellar and one cerebral cortical site, via closed-loop connections. We offer evidence supporting a new perspective that assigns the cerebellum the role of a coordinator of communication. We propose that the cerebellum participates in cognitive function by modulating the coherence of neuronal oscillations to optimize communications between multiple cortical structures in a task specific manner.
Keywords: cerebellum; cerebrocerebellar communication; cognition; coherence; functional connectivity.
Copyright © 2022 McAfee, Liu, Sillitoe and Heck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Cerebellar control of thalamocortical circuits for cognitive function: A review of pathways and a proposed mechanism.Front Syst Neurosci. 2023 Mar 30;17:1126508. doi: 10.3389/fnsys.2023.1126508. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37064161 Free PMC article. Review.
-
Cerebellar Lobulus Simplex and Crus I Differentially Represent Phase and Phase Difference of Prefrontal Cortical and Hippocampal Oscillations.Cell Rep. 2019 May 21;27(8):2328-2334.e3. doi: 10.1016/j.celrep.2019.04.085. Cell Rep. 2019. PMID: 31116979 Free PMC article.
-
The human cerebro-cerebellar system: its computing, cognitive, and language skills.Behav Brain Res. 1991 Aug 29;44(2):113-28. doi: 10.1016/s0166-4328(05)80016-6. Behav Brain Res. 1991. PMID: 1751002 Review.
-
Altered cortical-cerebellar circuits during verbal working memory in essential tremor.Brain. 2011 Aug;134(Pt 8):2274-86. doi: 10.1093/brain/awr164. Epub 2011 Jul 11. Brain. 2011. PMID: 21747127
-
[Role of the cerebellum in cognitive and behavioural control: scientific basis and investigation models].Acta Med Port. 2006 May-Jun;19(3):257-67. Epub 2006 Sep 7. Acta Med Port. 2006. PMID: 17234089 Review. Portuguese.
Cited by
-
Cerebellar control of fear learning via the cerebellar nuclei-Multiple pathways, multiple mechanisms?Front Syst Neurosci. 2023 May 9;17:1176668. doi: 10.3389/fnsys.2023.1176668. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37229350 Free PMC article. Review.
-
The cerebellum regulates fear extinction through thalamo-prefrontal cortex interactions in male mice.Nat Commun. 2023 Mar 17;14(1):1508. doi: 10.1038/s41467-023-36943-w. Nat Commun. 2023. PMID: 36932068 Free PMC article.
-
Secondary cerebro-cerebellar and intra-cerebellar dysfunction in cerebellar mutism syndrome.Neuro Oncol. 2024 Sep 5;26(9):1700-1711. doi: 10.1093/neuonc/noae070. Neuro Oncol. 2024. PMID: 38581226 Free PMC article.
-
Cerebellar mutism is linked to midbrain volatility and desynchronization from speech cortices.Brain. 2023 Nov 2;146(11):4755-4765. doi: 10.1093/brain/awad209. Brain. 2023. PMID: 37343136 Free PMC article.
-
Converging and Diverging Cerebellar Pathways for Motor and Social Behaviors in Mice.Cerebellum. 2024 Oct;23(5):1754-1767. doi: 10.1007/s12311-024-01706-w. Epub 2024 May 23. Cerebellum. 2024. PMID: 38780757 Free PMC article. Review.
References
-
- Andreasen N. C., O’Leary D. S., Cizadlo T., Arndt S., Rezai K., Ponto L. L., et al. (1996). Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc. Natl. Acad. Sci. U.S.A. 93 9985–9990. 10.1073/pnas.93.18.9985 - DOI - PMC - PubMed
-
- Andreasen N. C., Paradiso S., O’Leary D. S. (1998). “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull. 24 203–218. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

