Correlation Between Antimicrobial Resistance, Virulence Determinants and Biofilm Formation Ability Among Extraintestinal Pathogenic Escherichia coli Strains Isolated in Catalonia, Spain
- PMID: 35087504
- PMCID: PMC8786794
- DOI: 10.3389/fmicb.2021.803862
Correlation Between Antimicrobial Resistance, Virulence Determinants and Biofilm Formation Ability Among Extraintestinal Pathogenic Escherichia coli Strains Isolated in Catalonia, Spain
Abstract
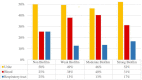
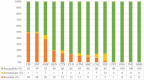
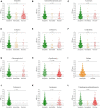
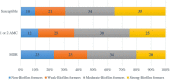
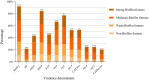
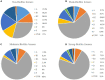
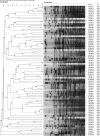
Escherichia coli is a well-characterized bacterium highly prevalent in the human intestinal tract and the cause of many important infections. The aim of this study was to characterize 376 extraintestinal pathogenic E. coli strains collected from four hospitals in Catalonia (Spain) between 2016 and 2017 in terms of antimicrobial resistance, siderophore production, phylogroup classification, and the presence of selected virulence and antimicrobial resistance genes. In addition, the association between these characteristics and the ability to form biofilms was also analyzed. The strains studied were classified into four groups according to their biofilm formation ability: non-biofilm formers (15.7%), weak (23.1%), moderate (35.6%), and strong biofilm formers (25.6%). The strains were highly resistant to ciprofloxacin (48.7%), trimethoprim-sulfamethoxazole (47.9%), and ampicillin (38%), showing a correlation between higher resistance to ciprofloxacin and lower biofilm production. Seventy-three strains (19.4%) were ESBL-producers. However, no relationship between the presence of ESBL and biofilm formation was found. The virulence factor genes fimH (92%), pgaA (84.6%), and irp1 (77.1%) were the most prevalent in all the studied strains. A statistically significant correlation was found between biofilm formation and the presence of iroN, papA, fimH, sfa, cnf, hlyA, iutA, and colibactin-encoding genes clbA, clbB, clbN, and clbQ. Interestingly, a high prevalence of colibactin-encoding genes (19.9%) was observed. Colibactin is a virulence factor, which interferes with the eukaryotic cell cycle and has been associated with colorectal cancer in humans. Most colibactin-encoding E. coli isolates belonged to phylogroup B2, exhibited low antimicrobial resistance but moderate or high biofilm-forming ability, and were significantly associated with most of the virulence factor genes tested. Additionally, the analysis of their clonal relatedness by PFGE showed 48 different clusters, indicating a high clonal diversity among the colibactin-positive strains. Several studies have correlated the pathogenicity of E. coli and the presence of virulence factor genes; however, colibactin and its relationship to biofilm formation have been scarcely investigated. The increasing prevalence of colibactin in E. coli and other Enterobacteriaceae and the recently described correlation with biofilm formation, makes colibactin a promising therapeutic target to prevent biofilm formation and its associated adverse effects.
Keywords: Escherichia coli; antimicrobial resistance; biofilm; colibactin; virulence.
Copyright © 2022 Ballén, Gabasa, Ratia, Sánchez and Soto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

