Pleckstrin Levels Are Increased in Patients with Chronic Periodontitis and Regulated via the MAP Kinase-p38α Signaling Pathway in Gingival Fibroblasts
- PMID: 35087525
- PMCID: PMC8787058
- DOI: 10.3389/fimmu.2021.801096
Pleckstrin Levels Are Increased in Patients with Chronic Periodontitis and Regulated via the MAP Kinase-p38α Signaling Pathway in Gingival Fibroblasts
Abstract
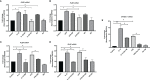
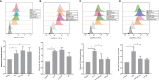
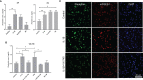
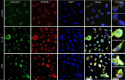
Chronic periodontitis (CP) is a bacteria-driven inflammatory disease characterized by the breakdown of gingival tissue, the periodontal ligament, and alveolar bone, leading ultimately to tooth loss. We previously reported the pleckstrin gene (PLEK) to be highly upregulated in gingival tissue of patients with CP and the only gene concurrently upregulated in other inflammatory diseases including rheumatoid arthritis and cardiovascular diseases. Using saliva from 169 individuals diagnosed with CP and healthy controls, we investigated whether pleckstrin could serve as a novel biomarker of periodontitis. Additionally, we explored signal pathways involved in the regulation of PLEK using human gingival fibroblasts (HGFs). Pleckstrin levels were significantly higher (p < 0.001) in the saliva samples of patients with CP compared to controls and closely associated with CP severity. Immunohistochemical analysis revealed the expression of pleckstrin in inflammatory cells and gingival fibroblasts of CP patients. To explore the signal pathways involved in pleckstrin regulation, we stimulated HGFs with either interleukin-1β (IL-1β) or lipopolysaccharides (LPS) alone, or in combination with inhibitors targeting c-Jun N-terminal kinase, tyrosine kinase, protein kinase C, or p38 MAP kinase. Results showed that IL-1β and LPS significantly increased PLEK mRNA and pleckstrin protein levels. VX-745, the p38 MAP kinase inhibitor significantly decreased IL-1β- and LPS-induced pleckstrin levels at both the mRNA and the protein level. Together, these findings show that pleckstrin could serve as a salivary biomarker for the chronic inflammatory disease periodontitis and a regulator of inflammation via the p38 MAP kinase pathway.
Keywords: MAP kinase pathway; PLEK; chronic periodontitis; gingival fibroblasts; inflammation; mPGES-1; pleckstrin.
Copyright © 2022 Alim, Njenda, Lundmark, Kaminska, Jansson, Eriksson, Kats, Johannsen, Arvidsson, Mydel and Yucel-Lindberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

