H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells
- PMID: 35087672
- PMCID: PMC8788236
- DOI: 10.1093/nsr/nwab137
H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells
Abstract
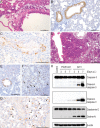
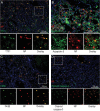
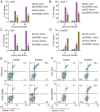
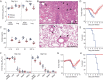
The H7N9 influenza virus emerged in China in 2013, causing more than 1560 human infections, 39% of which were fatal. A 'cytokine storm' in the lungs of H7N9 patients has been linked to a poor prognosis and death; however, the underlying mechanism that triggers the cytokine storm is unknown. Here, we found that efficient replication of the H7N9 virus in mouse lungs activates gasdermin E (GSDME)-mediated pyroptosis in alveolar epithelial cells, and that the released cytosolic contents then trigger a cytokine storm. Knockout of Gsdme switched the manner of death of A549 and human primary alveolar epithelial cells from pyroptosis to apoptosis upon H7N9 virus infection, and Gsdme knockout mice survived H7N9 virus lethal infection. Our findings reveal that GSDME activation is a key and unique mechanism for the pulmonary cytokine storm and lethal outcome of H7N9 virus infection and thus opens a new door for the development of antivirals against the H7N9 virus.
Keywords: H7N9 virus; cytokine storm; gasdermin E; pyroptosis.
© The Author(s) 2021. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures


References
LinkOut - more resources
Full Text Sources
