Tumor- and Osteoblast-Derived Periostin in Prostate Cancer bone Metastases
- PMID: 35087756
- PMCID: PMC8787093
- DOI: 10.3389/fonc.2021.795712
Tumor- and Osteoblast-Derived Periostin in Prostate Cancer bone Metastases
Abstract
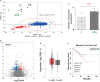
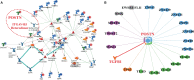
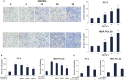
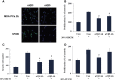
Exploring the biological function of periostin (POSTN) in prostate cancer (PCa) bone metastasis is of importance. It was observed that the expression of POSTN was high in PCa, especially highest in PCa metastasized to bone. In this study, we found that inhibiting POSTN in PCa cells could significantly alleviate PCa bone metastasis in vivo, suggesting POSTN is a promising therapeutic target. Since, due to the secreted expression of POSTN in osteoblasts and PCa, we hypothesized the positive feedback loop between osteoblasts and PCa mediated by POSTN in PCa bone metastasis. The in vitro experiments demonstrated that osteoblast-derived POSTN promoted PCa cell proliferation and invasion and PCa cell-derived POSTN promotes proliferation of osteoblasts. Furthermore, we found that POSTN regulated PCa and osteoblast function through integrin receptors. Finally, 18F-Alfatide II was used as the molecule probe of integrin αvβ3 in PET-CT, revealing high intake in metastatic lesions. Our findings together indicate that targeting POSTN in PCa cells as well as in the osteoblastic may be an effective treatment for PCa bone metastasis.
Keywords: bone metastasis; integrin receptor; osteoblast; periostin; prostate cancer.
Copyright © 2022 Sun, Mi, Ge, Hu, Xu, Guo, Tan, Zhang, Zhong and Xia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Fragkoulis C, Gkialas I, Papadopoulos G, Ntoumas K. Current Therapeutic Options Targeting Bone Metastasis in Metastatic Castration Resistant Prostate Cancer. J Buon (2016) 21(4):787–91. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

