Deep Metabolomic Profiling Reveals Alterations in Fatty Acid Synthesis and Ketone Body Degradations in Spermatozoa and Seminal Plasma of Astheno-Oligozoospermic Bulls
- PMID: 35087889
- PMCID: PMC8787163
- DOI: 10.3389/fvets.2021.755560
Deep Metabolomic Profiling Reveals Alterations in Fatty Acid Synthesis and Ketone Body Degradations in Spermatozoa and Seminal Plasma of Astheno-Oligozoospermic Bulls
Abstract
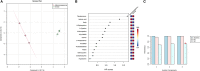
Male fertility is extremely important in dairy animals because semen from a single bull is used to inseminate several thousand females. Asthenozoospermia (reduced sperm motility) and oligozoospermia (reduced sperm concentration) are the two important reasons cited for idiopathic infertility in crossbred bulls; however, the etiology remains elusive. In this study, using a non-targeted liquid chromatography with tandem mass spectrometry-based approach, we carried out a deep metabolomic analysis of spermatozoa and seminal plasma derived from normozoospermic and astheno-oligozoospermic bulls. Using bioinformatics tools, alterations in metabolites and metabolic pathways between normozoospermia and astheno-oligozoospermia were elucidated. A total of 299 and 167 metabolites in spermatozoa and 183 and 147 metabolites in seminal plasma were detected in astheno-oligozoospermic and normozoospermic bulls, respectively. Among the mapped metabolites, 75 sperm metabolites were common to both the groups, whereas 166 and 50 sperm metabolites were unique to astheno-oligozoospermic and normozoospermic bulls, respectively. Similarly, 86 metabolites were common to both the groups, whereas 45 and 37 seminal plasma metabolites were unique to astheno-oligozoospermic and normozoospermic bulls, respectively. Among the differentially expressed metabolites, 62 sperm metabolites and 56 seminal plasma metabolites were significantly dysregulated in astheno-oligozoospermic bulls. In spermatozoa, selenocysteine, deoxyuridine triphosphate, and nitroprusside showed significant enrichment in astheno-oligozoospermic bulls. In seminal plasma, malonic acid, 5-diphosphoinositol pentakisphosphate, D-cysteine, and nicotinamide adenine dinucleotide phosphate were significantly upregulated, whereas tetradecanoyl-CoA was significantly downregulated in the astheno-oligozoospermia. Spermatozoa from astheno-oligozoospermic bulls showed alterations in the metabolism of fatty acid and fatty acid elongation in mitochondria pathways, whereas seminal plasma from astheno-oligozoospermic bulls showed alterations in synthesis and degradation of ketone bodies, pyruvate metabolism, and inositol phosphate metabolism pathways. The present study revealed vital information related to semen metabolomic differences between astheno-oligozoospermic and normospermic crossbred breeding bulls. It is inferred that fatty acid synthesis and ketone body degradations are altered in the spermatozoa and seminal plasma of astheno-oligozoospermic crossbred bulls. These results open up new avenues for further research, and current findings can be applied for the modulation of identified pathways to restore sperm motility and concentration in astheno-oligozoospermic bulls.
Keywords: dairy bulls; mass spectrometry; metabolomics; semen quality; seminal plasma; spermatozoa.
Copyright © 2022 Dasgupta, Kumaresan, Saraf, Nag, Sinha, Aslam M. K., Karthikkeyan, Prasad, Modi, Datta, Ramesha, Manimaran and Jeyakumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Mukhopadhyay CS, Gupta AK, Yadav BR, Khate K, Raina VS, Mohanty TK, et al. . Subfertility in males: an important cause of bull disposal in Bovines. Asian-Australasian J Anim Sci. (2010) 23:450–5. 10.5713/ajas.2010.90298 - DOI
-
- Mandal DK, Tyagi S, Ganguly I, Kumar S, Gaur GK, Tamilnadu J. Pattern of reproductive wastage and in Mandal, D. K, S Tyagi, I Ganguly, S Kumar, G K Gaur, and J Tamilnadu “Pattern of reproductive wastage and inheritance of semen quality in Frieswal crossbred bulls.” Tamilnadu J Vet Anim Sci. (2012) 8:245–9. Available online at: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.1071.8516&rep=...
LinkOut - more resources
Full Text Sources

