Effect of silver in thermal treatments of Fe-Mn-C degradable metals: Implications for stent processing
- PMID: 35087961
- PMCID: PMC8777259
- DOI: 10.1016/j.bioactmat.2021.10.020
Effect of silver in thermal treatments of Fe-Mn-C degradable metals: Implications for stent processing
Abstract
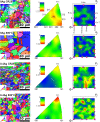
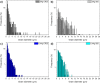
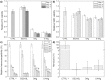
Twinning-induced plasticity (TWIP) steels are considered excellent materials for manufacturing products requiring extremely high mechanical properties for various applications including thin medical devices, such as biodegradable intravascular stents. It is also proven that the addition of Ag can guarantee an appropriate degradation while implanted in human body without affecting its bioactive properties. In order to develop an optimized manufacturing process for thin stents, the effect of Ag on the recrystallization behavior of TWIP steels needs to be elucidated. This is of major importance since manufacturing stents involves several intermediate recrystallization annealing treatments. In this work, the recrystallization mechanism of two Fe-Mn-C steels with and without Ag was thoroughly investigated by microstructural and mechanical analyses. It was observed that Ag promoted a finer microstructure with a different texture evolution, while the recrystallization kinetics resulted unaffected. The presence of Ag also reduced the effectiveness of the recrystallization treatment. This behavior was attributed to the presence of Ag-rich second phase particles, precipitation of carbides and to the preferential development of grains possessing a {111} orientation upon thermal treatment. The prominence of {111} grains can also give rise to premature twinning, explaining the role of Ag in reducing the ductility of TWIP steels already observed in other works. Furthermore, in vitro biological performances were unaffected by Ag. These findings could allow the design of efficient treatments for supporting the transformation of Fe-Mn-C steels alloyed with Ag into commercial products.
Keywords: Biomaterials; Recrystallization; Silver; Steels; Twinning.
© 2021 The Authors.
Conflict of interest statement
Dear Dr Yufeng Zheng, We hereby declare that this manuscript has not been published and it is not under consideration for publication elsewhere. We have no conflicts of interest to disclose.
Figures









References
-
- Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. doi: 10.1016/j.mser.2014.01.001. - DOI
-
- Garcia-Garcia H.M., Haude M., Kuku K., Hideo-Kajita A., Ince H., Abizaid A., Tölg R., Lemos P.A., von Birgelen C., Christiansen E.H., Wijns W., Escaned J., Dijkstra J., Waksman R. In vivo serial invasive imaging of the second-generation drug-eluting absorbable metal scaffold (Magmaris — DREAMS 2G) in de novo coronary lesions: insights from the BIOSOLVE-II First-In-Man Trial. Int. J. Cardiol. 2018;255:22–28. doi: 10.1016/j.ijcard.2017.12.053. - DOI - PubMed
-
- Haude M., Ince H., Abizaid A., Toelg R., Lemos P.A., von Birgelen C., Christiansen E.H., Wijns W., Neumann F.-J., Kaiser C., Eeckhout E., Lim S.T., Escaned J., Garcia-Garcia H.M., Waksman R. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016;387:31–39. doi: 10.1016/S0140-6736(15)00447-X. - DOI - PubMed
-
- Haude M., Ince H., Kische S., Abizaid A., Tölg R., Lemos P.A., Van Mieghem N.M., Verheye S., Von Birgelen C., Christiansen E.H., Wijns W., Garcia-Garcia H.M., Waksman R. Sustained safety and clinical performance of a drug-eluting absorbable metal scaffold up to 24 months: pooled outcomes of BIOSOLVE-II and BIOSOLVE-III. EuroIntervention. 2017;13:432–439. doi: 10.4244/EIJ-D-17-00254. - DOI - PubMed
LinkOut - more resources
Full Text Sources

