Effects of patient-reported outcome assessment order
- PMID: 35088616
- PMCID: PMC9232855
- DOI: 10.1177/17407745211073788
Effects of patient-reported outcome assessment order
Abstract
Background: In clinical trials and clinical practice, patient-reported outcomes are almost always assessed using multiple patient-reported outcome measures at the same time. This raises concerns about whether patient responses are affected by the order in which the patient-reported outcome measures are administered.
Methods: This questionnaire-based study of order effects included adult cancer patients from five cancer centers. Patients were randomly assigned to complete questionnaires via paper booklets, interactive voice response system, or tablet web survey. Linear Analogue Self-Assessment, Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events, and Patient-Reported Outcomes Measurement Information System assessment tools were each used to measure general health, physical function, social function, emotional distress/anxiety, emotional distress/depression, fatigue, sleep, and pain. The order in which the three tools, and domains within tools, were presented to patients was randomized. Rates of missing data, scale scores, and Cronbach's alpha coefficients were compared by the order in which they were assessed. Analyses included Cochran-Armitage trend tests and mixed models adjusted for performance score, age, sex, cancer type, and curative intent.
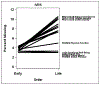
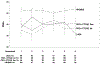
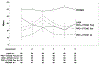
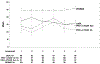
Results: A total of 1830 patients provided baseline patient-reported outcome assessments. There were no significant trends in rates of missing values by whether a scale was assessed earlier or later. The largest order effect for scale scores was due to a large mean score at one assessment time point. The largest difference in Cronbach's alpha between the versions for the Patient-Reported Outcomes Measurement Information System scales was 0.106.
Conclusion: The well-being of a cancer patient has many different aspects such as pain, fatigue, depression, and anxiety. These are assessed using a variety of surveys often collected at the same time. This study shows that the order in which the different aspects are collected from the patient is not important.
Keywords: Assessment order; LASA; PRO-CTCAE; PROMIS.
Conflict of interest statement
Declaration of competing interests
David Cella is a board member and officer of the PROMIS Health Organization, a 501(c)(3) charitable organization. All other authors have no conflicts of interest to report.
Figures
References
-
- Mccoll E, Eccles MP, Rousseau NS, et al. From the generic to the condition-specific?: instrument order effects in quality of life assessment. Med Care 2003; 41(7): 777–790. - PubMed
-
- Streiner DL and Norman GR. Health Measurement Scales: a practical guide to their development and use. 4th ed. Oxford University Press, 2008. pp. 108–110.
-
- Kapelner A and Chandler D. Preventing satisficing in online surveys. Proceedings of CrowdConf 2010.
-
- Yount S, List M, Du H, et al. A randomized validation study comparing embedded versus extracted FACT Head and Neck Symptom Index scores. Qual Life Res 2007; 16(10): 1615–1626. - PubMed
-
- Bretscher M, Rummans T, Sloan J, et al. Quality of life in hospice patients: a pilot study. Psychosomatics 1999; 40(4): 309–313. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

