Stem cell-derived sensory neurons modelling inherited erythromelalgia: normalization of excitability
- PMID: 35088838
- PMCID: PMC10060693
- DOI: 10.1093/brain/awac031
Stem cell-derived sensory neurons modelling inherited erythromelalgia: normalization of excitability
Abstract
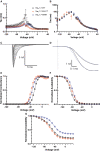
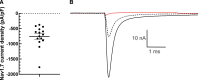
Effective treatment of pain remains an unmet healthcare need that requires new and effective therapeutic approaches. NaV1.7 has been genetically and functionally validated as a mediator of pain. Preclinical studies of NaV1.7-selective blockers have shown limited success and translation to clinical studies has been limited. The degree of NaV1.7 channel blockade necessary to attenuate neuronal excitability and ameliorate pain is an unanswered question important for drug discovery. Here, we utilize dynamic clamp electrophysiology and induced pluripotent stem cell-derived sensory neurons (iPSC-SNs) to answer this question for inherited erythromelalgia, a pain disorder caused by gain-of-function mutations in Nav1.7. We show that dynamic clamp can produce hyperexcitability in iPSC-SNs associated with two different inherited erythromelalgia mutations, NaV1.7-S241T and NaV1.7-I848T. We further show that blockade of approximately 50% of NaV1.7 currents can reverse neuronal hyperexcitability to baseline levels.
Keywords: Inherited erythromelalgia; Nav1.7; dynamic clamp; iPSC; stem cell-derived sensory neurons.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors report no competing interests.
Figures




References
-
- Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. - PubMed
-
- Elzahaf RA, Tashani OA, Unsworth BA, Johnson MI. The prevalence of chronic pain with an analysis of countries with a Human Development Index less than 0.9: a systematic review without meta-analysis. Curr Med Res Opin. 2012;28(7):1221–9. - PubMed
-
- Menefee LA, Frank ED, Doghramji K, et al. Self-reported sleep quality and quality of life for individuals with chronic pain conditions. Clin J Pain. 2000;16(4):290–297. - PubMed

