Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo
- PMID: 35089096
- PMCID: PMC8725587
- DOI: 10.1128/mBio.03144-21
Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo
Abstract
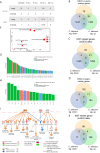
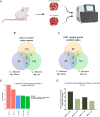
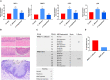
Oral squamous cell carcinoma (OSCC) is associated with oral Candida albicans infection, although it is unclear whether the fungus promotes the genesis and progression of OSCC or whether cancer facilitates fungal growth. In this study, we investigated whether C. albicans can potentiate OSCC tumor development and progression. In vitro, the presence of live C. albicans, but not Candida parapsilosis, enhanced the progression of OSCC by stimulating the production of matrix metalloproteinases, oncometabolites, protumor signaling pathways, and overexpression of prognostic marker genes associated with metastatic events. C. albicans also upregulated oncogenes in nonmalignant cells. Using a newly established xenograft in vivo mouse model to investigate OSCC-C. albicans interactions, oral candidiasis enhanced the progression of OSCC through inflammation and induced the overexpression of metastatic genes and significant changes in markers of the epithelial-mesenchymal transition. Finally, using the 4-nitroquinoline 1-oxide (4NQO) murine model, we directly correlate these in vitro and short-term in vivo findings with the progression of oncogenesis over the long term. Taken together, these data indicate that C. albicans upregulates oncogenes, potentiates a premalignant phenotype, and is involved in early and late stages of malignant promotion and progression of oral cancer. IMPORTANCE Oral squamous cell carcinoma (OSCC) is a serious health issue worldwide that accounts for 2% to 4% of all cancer cases. Previous studies have revealed a higher yeast carriage and diversity in oral cancer patients than in healthy individuals. Furthermore, fungal colonization in the oral cavity bearing OSCC is higher on the neoplastic epithelial surface than on adjacent healthy surfaces, indicating a positive association between oral yeast carriage and epithelial carcinoma. In addition to this, there is strong evidence supporting the idea that Candida contributes to carcinogenesis events in the oral cavity. Here, we show that an increase in Candida albicans burden promotes an oncogenic phenotype in the oral cavity.
Keywords: Candida albicans; cancer; oral squamous cell carcinoma; progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Diz P, Meleti M, Diniz-Freitas M, Vescovi P, Warnakulasuriya S, Johnson NW, Kerr AR. 2017. Oral and pharyngeal cancer in Europe: incidence, mortality and trends as presented to the Global Oral Cancer Forum. Transl Res Oral Oncol 2:2057178X1770151. doi:10.1177/2057178X17701517. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
