Role of glycosyltransferases in carcinogenesis; growth factor signaling and EMT/MET programs
- PMID: 35089466
- PMCID: PMC8795723
- DOI: 10.1007/s10719-022-10041-3
Role of glycosyltransferases in carcinogenesis; growth factor signaling and EMT/MET programs
Abstract
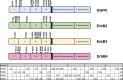
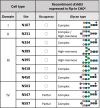
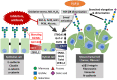
The glycosylation of cell surface receptors has been shown to regulate each step of signal transduction, including receptor trafficking to the cell surface, ligand binding, dimerization, phosphorylation, and endocytosis. In this review we focus on the role of glycosyltransferases that are involved in the modification of N-glycans, such as the effect of branching and elongation in signaling by various cell surface receptors. In addition, the role of those enzymes in the EMT/MET programs, as related to differentiation and cancer development, progress and therapy resistance is discussed.
Keywords: Cell surface receptor; Collectin; EGFR; EMT programs; ErbB receptors; Fut8; GM3; GnT-III; GnT-V; ST6Gal1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Hakomori S, Handa K, Iwabuchi K, Yamamura S, Prinetti A. New insights in glycosphingolipid function: "glycosignaling domain," a cell surface assembly of glycosphingolipids with signal transducer molecules, involved in cell adhesion coupled with signaling. Glycobiology. 1998;8(10):xi–xix. doi: 10.1093/oxfordjournals.glycob.a018822. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

