Synthesis of Natural Products by C-H Functionalization of Heterocycless
- PMID: 35089624
- PMCID: PMC9035081
- DOI: 10.1002/chem.202104278
Synthesis of Natural Products by C-H Functionalization of Heterocycless
Abstract
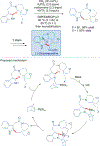
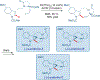
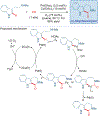
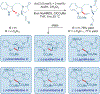
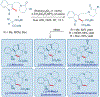
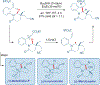
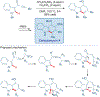
Total synthesis is considered by many as the finest combination of art and science. During the last decades, several concepts were proposed for achieving the perfect vision of total synthesis, such as atom economy, step economy, or redox economy. In this context, C-H functionalization represents the most powerful platform that has emerged in the last years, empowering rapid synthesis of complex natural products and enabling diversification of bioactive scaffolds based on natural product architectures. In this review, we present an overview of the recent strategies towards the total synthesis of heterocyclic natural products enabled by C-H functionalization. Heterocycles represent the most common motifs in drug discovery and marketed drugs. The implementation of C-H functionalization of heterocycles enables novel tactics in the construction of core architectures, but also changes the logic design of retrosynthetic strategies and permits access to natural product scaffolds with novel and enhanced biological activities.
Keywords: C−H activation; C−H functionalization; heterocycles; natural products; total synthesis.
© 2022 Wiley-VCH GmbH.
Figures



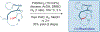












References
-
- Nicolaou KC, Vourloumis D, Winssinger N, Baran PS, Angew. Chem. Int. Ed 2000, 39, 44–122; Angew. Chem. 2000, 112, 46–126. - PubMed
-
- Corey EJ, Cheng XM, The Logic of Chemical Synthesis; Wiley, New York, 1989;
- Nicolaou KC, Sorensen EJ, Classics in Total Synthesis: Targets, Strategies, Methods; Wiley, Weinheim, 1996;
- Nicolaou KC, Snyder SA, Classics in Total Synthesis II: More Targets, Strategies, Methods; Wiley, Weinheim, 2003.
-
- For divergent total synthesis, see:
- Li L, Chen Z, Zhang X, Jia Y, Chem. Rev 2018, 118, 3752–3832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

