A high-throughput method to deliver targeted optogenetic stimulation to moving C. elegans populations
- PMID: 35089912
- PMCID: PMC8827482
- DOI: 10.1371/journal.pbio.3001524
A high-throughput method to deliver targeted optogenetic stimulation to moving C. elegans populations
Update in
-
Inhibitory feedback from the motor circuit gates mechanosensory processing in Caenorhabditis elegans.PLoS Biol. 2023 Sep 21;21(9):e3002280. doi: 10.1371/journal.pbio.3002280. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37733772 Free PMC article.
Abstract
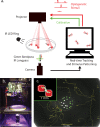
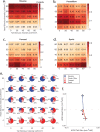
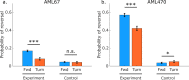
We present a high-throughput optogenetic illumination system capable of simultaneous closed-loop light delivery to specified targets in populations of moving Caenorhabditis elegans. The instrument addresses three technical challenges: It delivers targeted illumination to specified regions of the animal's body such as its head or tail; it automatically delivers stimuli triggered upon the animal's behavior; and it achieves high throughput by targeting many animals simultaneously. The instrument was used to optogenetically probe the animal's behavioral response to competing mechanosensory stimuli in the the anterior and posterior gentle touch receptor neurons. Responses to more than 43,418 stimulus events from a range of anterior-posterior intensity combinations were measured. The animal's probability of sprinting forward in response to a mechanosensory stimulus depended on both the anterior and posterior stimulation intensity, while the probability of reversing depended primarily on the anterior stimulation intensity. We also probed the animal's response to mechanosensory stimulation during the onset of turning, a relatively rare behavioral event, by delivering stimuli automatically when the animal began to turn. Using this closed-loop approach, over 9,700 stimulus events were delivered during turning onset at a rate of 9.2 events per worm hour, a greater than 25-fold increase in throughput compared to previous investigations. These measurements validate with greater statistical power previous findings that turning acts to gate mechanosensory evoked reversals. Compared to previous approaches, the current system offers targeted optogenetic stimulation to specific body regions or behaviors with many fold increases in throughput to better constrain quantitative models of sensorimotor processing.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Inhibitory feedback from the motor circuit gates mechanosensory processing in Caenorhabditis elegans.PLoS Biol. 2023 Sep 21;21(9):e3002280. doi: 10.1371/journal.pbio.3002280. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37733772 Free PMC article.
-
The tactile receptive fields of freely moving Caenorhabditis elegans nematodes.Integr Biol (Camb). 2018 Aug 1;10(8):450-463. doi: 10.1039/c8ib00045j. Epub 2018 Jul 20. Integr Biol (Camb). 2018. PMID: 30027970 Free PMC article.
-
Comparing Caenorhabditis elegans gentle and harsh touch response behavior using a multiplexed hydraulic microfluidic device.Integr Biol (Camb). 2017 Oct 16;9(10):800-809. doi: 10.1039/c7ib00120g. Integr Biol (Camb). 2017. PMID: 28914311 Free PMC article.
-
How Caenorhabditis elegans Senses Mechanical Stress, Temperature, and Other Physical Stimuli.Genetics. 2019 May;212(1):25-51. doi: 10.1534/genetics.118.300241. Genetics. 2019. PMID: 31053616 Free PMC article. Review.
-
The whole worm: brain-body-environment models of C. elegans.Curr Opin Neurobiol. 2016 Oct;40:23-30. doi: 10.1016/j.conb.2016.06.005. Epub 2016 Jun 20. Curr Opin Neurobiol. 2016. PMID: 27336738 Review.
Cited by
-
TWISP: A Transgenic Worm for Interrogating Signal Propagation in C. elegans.bioRxiv [Preprint]. 2023 Aug 5:2023.08.03.551820. doi: 10.1101/2023.08.03.551820. bioRxiv. 2023. Update in: Genetics. 2024 Jul 8;227(3):iyae077. doi: 10.1093/genetics/iyae077. PMID: 37577580 Free PMC article. Updated. Preprint.
-
An inhibitory acetylcholine receptor gates context dependent mechanosensory processing in C. elegans.bioRxiv [Preprint]. 2024 Mar 27:2024.03.21.586204. doi: 10.1101/2024.03.21.586204. bioRxiv. 2024. Update in: iScience. 2024 Aug 22;27(10):110776. doi: 10.1016/j.isci.2024.110776. PMID: 38585821 Free PMC article. Updated. Preprint.
-
Long-term imaging reveals behavioral plasticity during C. elegans dauer exit.BMC Biol. 2022 Dec 13;20(1):277. doi: 10.1186/s12915-022-01471-4. BMC Biol. 2022. PMID: 36514066 Free PMC article.
-
Decoding sexual dimorphism of the sex-shared nervous system at single-neuron resolution.bioRxiv [Preprint]. 2025 May 8:2024.12.27.630541. doi: 10.1101/2024.12.27.630541. bioRxiv. 2025. Update in: Sci Adv. 2025 Jul 11;11(28):eadv9106. doi: 10.1126/sciadv.adv9106. PMID: 40654890 Free PMC article. Updated. Preprint.
-
Probiotic effects of Lactococcus lactis and Leuconostoc mesenteroides on stress and longevity in Caenorhabditis elegans.Front Physiol. 2023 Sep 12;14:1207705. doi: 10.3389/fphys.2023.1207705. eCollection 2023. Front Physiol. 2023. PMID: 37772058 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

