Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study
- PMID: 35090007
- PMCID: PMC9156377
- DOI: 10.1093/eurheartj/ehab921
Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study
Abstract
Aims: To report 5-year outcomes of EFFORTLESS registry patients with early generation subcutaneous implantable cardioverter-defibrillator (S-ICD) devices.
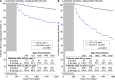
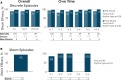
Methods and results: Kaplan-Meier, trend and multivariable analyses were performed for mortality and late (years 2-5) complications, appropriate shock (AS) and inappropriate shock (IAS) rates. Nine hundred and eighty-four of 994 enrolled patients with diverse diagnoses (28% female, 48 ± 17 years, body mass index 27 ± 6 kg/m2, ejection fraction 43 ± 18%) underwent S-ICD implantation. Median follow-up was 5.1 years (interquartile range 4.7-5.5 years). All-cause mortality was 9.3% (95% confidence interval 7.2-11.3%) at 5 years; 703 patients remained in follow-up on study completion, 171 withdrew including 87 (8.8%) with device explanted, and 65 (6.6%) lost to follow-up. Of the explants, only 20 (2.0%) patients needed a transvenous device for pacing indications. First and final shock efficacy for discrete ventricular arrhythmias was consistent at 90% and 98%, respectively, with storm episode final shock efficacy at 95.2%. Time to therapy remained unaltered. Overall 1- and 5-year complication rates were 8.9% and 15.2%, respectively. Early complications did not predict later complications. There were no structural lead failures. Inappropriate shock rates at 1 and 5 years were 8.7% and 16.9%, respectively. Self-terminating inappropriately sensed episodes predicted late IAS. Predictors of late AS included self-terminating appropriately sensed episodes and earlier AS.
Conclusion: In this diverse S-ICD registry population, spontaneous shock efficacy was consistently high over 5 years. Very few patients underwent S-ICD replacement with a transvenous device for pacing indications. Treated and self-terminating arrhythmic episodes predict future shock events, which should encourage more personalized device optimization.
Keywords: Implantable cardioverter-defibrillator; Primary prevention; Secondary prevention; Subcutaneous ICD; Sudden death.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Figures






Comment in
-
Long-term results of first-generation S-ICD systems: strong from the start and through the threats of time.Eur Heart J. 2022 Jun 1;43(21):2051-2053. doi: 10.1093/eurheartj/ehac137. Eur Heart J. 2022. PMID: 35362061 No abstract available.
References
-
- Boersma L, Barr C, Knops R, Theuns D, Eckardt L, Neuzil P, et al. Implant and midterm outcomes of the subcutaneous implantable cardioverter-defibrillator registry: the EFFORTLESS study. J Am Coll Cardiol 2017;70:830–841. - PubMed
-
- Pedersen SS, Lambiase P, Boersma LVA, Murgatroyd F, Johansen JB, Reeve H, et al. Evaluation oF FactORs ImpacTing CLinical Outcome and Cost EffectiveneSS of the S-ICD: design and rationale of the EFFORTLESS S-ICD Registry. Pacing Clin Electrophysiol 2012;35:574–579. - PubMed
-
- Boston Scientific Corporation . Product Advisories – Boston Scientific 2021 [Available from: https://www.bostonscientific.com/en-US/pprc/product-advisories.html.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

