Expanding Therapeutic Opportunities for Extrapulmonary Neuroendocrine Carcinoma
- PMID: 35091446
- PMCID: PMC7612728
- DOI: 10.1158/1078-0432.CCR-21-3058
Expanding Therapeutic Opportunities for Extrapulmonary Neuroendocrine Carcinoma
Abstract
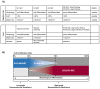
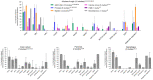
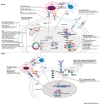
Poorly differentiated neuroendocrine carcinomas (PD-NEC) are rare cancers garnering interest as they become more commonly encountered in the clinic. This is due to improved diagnostic methods and the increasingly observed phenomenon of "NE lineage plasticity," whereby nonneuroendocrine (non-NE) epithelial cancers transition to aggressive NE phenotypes after targeted treatment. Effective treatment options for patients with PD-NEC are challenging for several reasons. This includes a lack of targetable, recurrent molecular drivers, a paucity of patient-relevant preclinical models to study biology and test novel therapeutics, and the absence of validated biomarkers to guide clinical management. Although advances have been made pertaining to molecular subtyping of small cell lung cancer (SCLC), a PD-NEC of lung origin, extrapulmonary (EP)-PD-NECs remain understudied. This review will address emerging SCLC-like, same-organ non-NE cancer-like and tumor-type-agnostic biological vulnerabilities of EP-PD-NECs, with the potential for therapeutic exploitation. The hypotheses surrounding the origin of these cancers and how "NE lineage plasticity" can be leveraged for therapeutic purposes are discussed. SCLC is herein proposed as a paradigm for supporting progress toward precision medicine in EP-PD-NECs. The aim of this review is to provide a thorough portrait of the current knowledge of EP-PD-NEC biology, with a view to informing new avenues for research and future therapeutic opportunities in these cancers of unmet need.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- WHO Classification of Tumours Editorial Board. Digestive System Tumours. Lyon (France): International Agency for Research in Cancer (IARC); 2019. WHO Classification of Tumours.
-
- Travis W, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon (France): International Agency for Research on Cancer (IARC); 2015. - PubMed
-
- Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86. doi: 10.1038/s41379-018-0110-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

