Valence opponency in peripheral olfactory processing
- PMID: 35091473
- PMCID: PMC8812543
- DOI: 10.1073/pnas.2120134119
Valence opponency in peripheral olfactory processing
Abstract
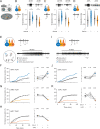
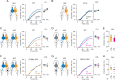
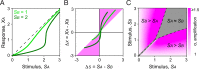
A hallmark of complex sensory systems is the organization of neurons into functionally meaningful maps, which allow for comparison and contrast of parallel inputs via lateral inhibition. However, it is unclear whether such a map exists in olfaction. Here, we address this question by determining the organizing principle underlying the stereotyped pairing of olfactory receptor neurons (ORNs) in Drosophila sensory hairs, wherein compartmentalized neurons inhibit each other via ephaptic coupling. Systematic behavioral assays reveal that most paired ORNs antagonistically regulate the same type of behavior. Such valence opponency is relevant in critical behavioral contexts including place preference, egg laying, and courtship. Odor-mixture experiments show that ephaptic inhibition provides a peripheral means for evaluating and shaping countervailing cues relayed to higher brain centers. Furthermore, computational modeling suggests that this organization likely contributes to processing ratio information in odor mixtures. This olfactory valence map may have evolved to swiftly process ethologically meaningful odor blends without involving costly synaptic computation.
Keywords: countervailing cues; olfactory receptor neurons (ORNs); olfactory sensilla; sensory map; valence opponency.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- van Hateren J. H., A theory of maximizing sensory information. Biol. Cybern. 68, 23–29 (1992). - PubMed
-
- Soucy E. R., Albeanu D. F., Fantana A. L., Murthy V. N., Meister M., Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 12, 210–220 (2009). - PubMed
-
- Hallem E. A., Carlson J. R., Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006). - PubMed
-
- Hallem E. A., Ho M. G., Carlson J. R., The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979 (2004). - PubMed
-
- Fishilevich E., Vosshall L. B., Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

