Neural Encoding of Active Multi-Sensing Enhances Perceptual Decision-Making via a Synergistic Cross-Modal Interaction
- PMID: 35091504
- PMCID: PMC8936614
- DOI: 10.1523/JNEUROSCI.0861-21.2022
Neural Encoding of Active Multi-Sensing Enhances Perceptual Decision-Making via a Synergistic Cross-Modal Interaction
Abstract
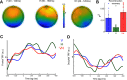
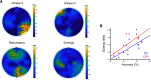
Most perceptual decisions rely on the active acquisition of evidence from the environment involving stimulation from multiple senses. However, our understanding of the neural mechanisms underlying this process is limited. Crucially, it remains elusive how different sensory representations interact in the formation of perceptual decisions. To answer these questions, we used an active sensing paradigm coupled with neuroimaging, multivariate analysis, and computational modeling to probe how the human brain processes multisensory information to make perceptual judgments. Participants of both sexes actively sensed to discriminate two texture stimuli using visual (V) or haptic (H) information or the two sensory cues together (VH). Crucially, information acquisition was under the participants' control, who could choose where to sample information from and for how long on each trial. To understand the neural underpinnings of this process, we first characterized where and when active sensory experience (movement patterns) is encoded in human brain activity (EEG) in the three sensory conditions. Then, to offer a neurocomputational account of active multisensory decision formation, we used these neural representations of active sensing to inform a drift diffusion model of decision-making behavior. This revealed a multisensory enhancement of the neural representation of active sensing, which led to faster and more accurate multisensory decisions. We then dissected the interactions between the V, H, and VH representations using a novel information-theoretic methodology. Ultimately, we identified a synergistic neural interaction between the two unisensory (V, H) representations over contralateral somatosensory and motor locations that predicted multisensory (VH) decision-making performance.SIGNIFICANCE STATEMENT In real-world settings, perceptual decisions are made during active behaviors, such as crossing the road on a rainy night, and include information from different senses (e.g., car lights, slippery ground). Critically, it remains largely unknown how sensory evidence is combined and translated into perceptual decisions in such active scenarios. Here we address this knowledge gap. First, we show that the simultaneous exploration of information across senses (multi-sensing) enhances the neural encoding of active sensing movements. Second, the neural representation of active sensing modulates the evidence available for decision; and importantly, multi-sensing yields faster evidence accumulation. Finally, we identify a cross-modal interaction in the human brain that correlates with multisensory performance, constituting a putative neural mechanism for forging active multisensory perception.
Keywords: EEG; active sensing; drift diffusion model; multisensory processing; partial information decomposition; perceptual decision-making.
Copyright © 2022 the authors.
Figures


Similar articles
-
Correlation of neural activity with behavioral kinematics reveals distinct sensory encoding and evidence accumulation processes during active tactile sensing.Neuroimage. 2018 Jul 15;175:12-21. doi: 10.1016/j.neuroimage.2018.03.035. Epub 2018 Mar 23. Neuroimage. 2018. PMID: 29580968 Free PMC article.
-
Neurocomputational mechanisms underlying cross-modal associations and their influence on perceptual decisions.Neuroimage. 2022 Feb 15;247:118841. doi: 10.1016/j.neuroimage.2021.118841. Epub 2021 Dec 21. Neuroimage. 2022. PMID: 34952232 Free PMC article.
-
The interplay between multisensory integration and perceptual decision making.Neuroimage. 2020 Nov 15;222:116970. doi: 10.1016/j.neuroimage.2020.116970. Epub 2020 May 23. Neuroimage. 2020. PMID: 32454204
-
Where are multisensory signals combined for perceptual decision-making?Curr Opin Neurobiol. 2016 Oct;40:31-37. doi: 10.1016/j.conb.2016.06.003. Epub 2016 Jun 23. Curr Opin Neurobiol. 2016. PMID: 27344253 Review.
-
Multisensory decisions from self to world.Philos Trans R Soc Lond B Biol Sci. 2023 Sep 25;378(1886):20220335. doi: 10.1098/rstb.2022.0335. Epub 2023 Aug 7. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37545311 Free PMC article. Review.
Cited by
-
Genuine high-order interactions in brain networks and neurodegeneration.Neurobiol Dis. 2022 Dec;175:105918. doi: 10.1016/j.nbd.2022.105918. Epub 2022 Nov 12. Neurobiol Dis. 2022. PMID: 36375407 Free PMC article.
-
A drift diffusion model analysis of age-related impact on multisensory decision-making processes.Sci Rep. 2024 Jun 28;14(1):14895. doi: 10.1038/s41598-024-65549-5. Sci Rep. 2024. PMID: 38942761 Free PMC article.
-
Quantifying the diverse contributions of hierarchical muscle interactions to motor function.iScience. 2024 Dec 16;28(1):111613. doi: 10.1016/j.isci.2024.111613. eCollection 2025 Jan 17. iScience. 2024. PMID: 39834869 Free PMC article.
-
Flexibility in choosing decision policies in gathering discrete evidence over time.PLoS One. 2025 Jan 14;20(1):e0316320. doi: 10.1371/journal.pone.0316320. eCollection 2025. PLoS One. 2025. PMID: 39808606 Free PMC article.
-
Intermodulation from Unisensory to Multisensory Perception: A Review.Brain Sci. 2022 Nov 25;12(12):1617. doi: 10.3390/brainsci12121617. Brain Sci. 2022. PMID: 36552077 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
