Mu Opioid Receptors Acutely Regulate Adenosine Signaling in Striatal Glutamate Afferents
- PMID: 35091505
- PMCID: PMC8944233
- DOI: 10.1523/JNEUROSCI.1039-21.2022
Mu Opioid Receptors Acutely Regulate Adenosine Signaling in Striatal Glutamate Afferents
Abstract
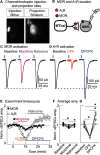
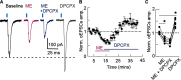
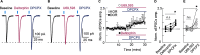
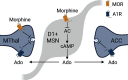
Endogenous adenosine plays a crucial role in maintaining energy homeostasis, and adenosine levels are tightly regulated across neural circuits. In the dorsal medial striatum (DMS), adenosine inhibits neurotransmitter release, but the source and mechanism underlying its accumulation are largely unknown. Opioids also inhibit neurotransmitter release in the DMS and influence adenosine accumulation after prolonged exposure. However, how these two neurotransmitter systems interact acutely is also largely unknown. This study demonstrates that activation of µ opioid receptors, but not δ opioid receptors or κ opioid receptors, inhibits tonic activation of adenosine A1Rs via a cAMP-dependent mechanism in both male and female mice. Further, selectively knocking out µ opioid receptors from thalamic presynaptic terminals and postsynaptic medium spiny neurons (MSNs) revealed that activation of µ opioid receptors on D1R-positive MSNs, but not D2R-positive MSNs, is necessary to inhibit tonic adenosine signaling on presynaptic terminals. Given the role of D1R-positive MSNs in movement and motivated behaviors, these findings reveal a novel mechanism by which these neurons regulate their own synaptic inputs.SIGNIFICANCE STATEMENT Understanding interactions between neuromodulatory systems within brain circuits is a fundamental question in neuroscience. The present work uncovers a novel role of opioids in acutely inhibiting adenosine accumulation and subsequent adenosine receptor signaling in the striatum by inhibiting the production of cAMP. Adenosine receptor signaling regulates striatal neurotransmitters, including glutamate, GABA, dopamine, and acetylcholine. Furthermore, interactions between adenosine2A receptors and numerous other GPCRs, including D2 dopamine and CB1 cannabinoid receptors, suggest that endogenous adenosine broadly modulates striatal GPCR signaling. Additionally, this work discovered that the source of resting endogenous extracellular adenosine is likely D1, but not D2 receptor-positive medium spiny neurons, suggesting that opioid signaling and manipulation of D1R-expressing medium spiny neuron cAMP activity can broadly affect striatal function and behavior.
Keywords: adenosine; opioids; striatum; synaptic transmission; thalamus.
Copyright © 2022 the authors.
Figures







References
-
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo CO, Park S, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR (2015) Distinct subpopulation of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87:1063–1077. 10.1016/j.neuron.2015.08.019 - DOI - PMC - PubMed
-
- Bertran-Gonzalez J, Laurent V, Chieng BC, Christie MJ, Balleine BW (2013) Learning-related translocation of delta-opioid receptors on ventral striatal cholinergic interneurons mediates choice between goal-directed actions. J Neurosci 33:16060–16071. 10.1523/JNEUROSCI.1927-13.2013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
