Inherited IFNAR1 Deficiency in a Child with Both Critical COVID-19 Pneumonia and Multisystem Inflammatory Syndrome
- PMID: 35091979
- PMCID: PMC8798309
- DOI: 10.1007/s10875-022-01215-7
Inherited IFNAR1 Deficiency in a Child with Both Critical COVID-19 Pneumonia and Multisystem Inflammatory Syndrome
Abstract
Background: Inborn errors of immunity (IEI) and autoantibodies to type I interferons (IFNs) underlie critical COVID-19 pneumonia in at least 15% of the patients, while the causes of multisystem inflammatory syndrome in children (MIS-C) remain elusive.
Objectives: To detect causal genetic variants in very rare cases with concomitant critical COVID-19 pneumonia and MIS-C.
Methods: Whole exome sequencing was performed, and the impact of candidate gene variants was investigated. Plasma levels of cytokines, specific antibodies against the virus, and autoantibodies against type I IFNs were also measured.
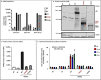
Results: We report a 3-year-old child who died on day 56 of SARS-CoV-2 infection with an unusual clinical presentation, combining both critical COVID-19 pneumonia and MIS-C. We identified a large, homozygous loss-of-function deletion in IFNAR1, underlying autosomal recessive IFNAR1 deficiency.
Conclusions: Our findings confirm that impaired type I IFN immunity can underlie critical COVID-19 pneumonia, while suggesting that it can also unexpectedly underlie concomitant MIS-C. Our report further raises the possibility that inherited or acquired dysregulation of type I IFN immunity might contribute to MIS-C in other patients.
Keywords: COVID-19; IFNAR1; critical pneumonia; inborn errors of immunity (IEI); multisystem inflammatory syndrome in children (MIS-C); primary immunodeficiency (PID).
© 2022. The Author(s).
Conflict of interest statement
J.L.C. reports being an inventor on patent application PCT/US2021/042741, filed 22 July 2021, submitted by The Rockefeller University, which covers the diagnosis of, susceptibility to, and treatment of viral disease and viral vaccines, including COVID-19 and vaccine-associated diseases. The other authors have no conflicts of interest to report.
Figures

References
-
- Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries. PLoS ONE. 2020;15(6):e0234765. doi: 10.1371/journal.pone.0234765. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

