Impaired photoprotection in Phaeodactylum tricornutum KEA3 mutants reveals the proton regulatory circuit of diatoms light acclimation
- PMID: 35092009
- PMCID: PMC9306478
- DOI: 10.1111/nph.18003
Impaired photoprotection in Phaeodactylum tricornutum KEA3 mutants reveals the proton regulatory circuit of diatoms light acclimation
Abstract
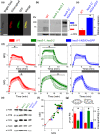
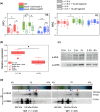
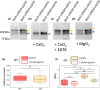
Diatoms are successful phytoplankton clades able to acclimate to changing environmental conditions, including e.g. variable light intensity. Diatoms are outstanding at dissipating light energy exceeding the maximum photosynthetic electron transfer (PET) capacity via the nonphotochemical quenching (NPQ) process. While the molecular effectors of NPQ as well as the involvement of the proton motive force (PMF) in its regulation are known, the regulators of the PET/PMF relationship remain unidentified in diatoms. We generated mutants of the H+ /K+ antiporter KEA3 in the model diatom Phaeodactylum tricornutum. Loss of KEA3 activity affects the PET/PMF coupling and NPQ responses at the onset of illumination, during transients and in steady-state conditions. Thus, this antiporter is a main regulator of the PET/PMF coupling. Consistent with this conclusion, a parsimonious model including only two free components, KEA3 and the diadinoxanthin de-epoxidase, describes most of the feedback loops between PET and NPQ. This simple regulatory system allows for efficient responses to fast (minutes) or slow (e.g. diel) changes in light environment, thanks to the presence of a regulatory calcium ion (Ca2+ )-binding domain in KEA3 modulating its activity. This circuit is likely tuned by the NPQ-effector proteins, LHCXs, providing diatoms with the required flexibility to thrive in different ocean provinces.
Keywords: diatoms; ion channels; nonphotochemical quenching; photosynthesis; phytoplankton; proton motive force.
© 2022 The Authors New Phytologist © 2022 New Phytologist Foundation.
Figures


Similar articles
-
The diatom Phaeodactylum tricornutum adjusts nonphotochemical fluorescence quenching capacity in response to dynamic light via fine-tuned Lhcx and xanthophyll cycle pigment synthesis.New Phytol. 2017 Apr;214(1):205-218. doi: 10.1111/nph.14337. Epub 2016 Nov 21. New Phytol. 2017. PMID: 27870063
-
Dynamic Changes between Two LHCX-Related Energy Quenching Sites Control Diatom Photoacclimation.Plant Physiol. 2018 Jul;177(3):953-965. doi: 10.1104/pp.18.00448. Epub 2018 May 17. Plant Physiol. 2018. PMID: 29773581 Free PMC article.
-
Multisignal control of expression of the LHCX protein family in the marine diatom Phaeodactylum tricornutum.J Exp Bot. 2016 Jun;67(13):3939-51. doi: 10.1093/jxb/erw198. Epub 2016 May 25. J Exp Bot. 2016. PMID: 27225826 Free PMC article.
-
Diversity in Xanthophyll Cycle Pigments Content and Related Nonphotochemical Quenching (NPQ) Among Microalgae: Implications for Growth Strategy and Ecology.J Phycol. 2020 Apr;56(2):245-263. doi: 10.1111/jpy.12944. Epub 2020 Feb 5. J Phycol. 2020. PMID: 31674660 Review.
-
Contribution of Cyclic and Pseudo-cyclic Electron Transport to the Formation of Proton Motive Force in Chloroplasts.Mol Plant. 2017 Jan 9;10(1):20-29. doi: 10.1016/j.molp.2016.08.004. Epub 2016 Aug 26. Mol Plant. 2017. PMID: 27575692 Review.
Cited by
-
Diatoms exhibit dynamic chloroplast calcium signals in response to high light and oxidative stress.Plant Physiol. 2024 Dec 23;197(1):kiae591. doi: 10.1093/plphys/kiae591. Plant Physiol. 2024. PMID: 39515781 Free PMC article.
-
A rapid aureochrome opto-switch enables diatom acclimation to dynamic light.Nat Commun. 2024 Jul 3;15(1):5578. doi: 10.1038/s41467-024-49991-7. Nat Commun. 2024. PMID: 38956103 Free PMC article.
-
Functional study of Phaeodactylum tricornutum Seipin highlights specificities of lipid droplets biogenesis in diatoms.New Phytol. 2025 Sep;247(5):2245-2269. doi: 10.1111/nph.70350. Epub 2025 Jul 7. New Phytol. 2025. PMID: 40619984 Free PMC article.
-
A metabolic, phylogenomic and environmental atlas of diatom plastid transporters from the model species Phaeodactylum.Front Plant Sci. 2022 Sep 22;13:950467. doi: 10.3389/fpls.2022.950467. eCollection 2022. Front Plant Sci. 2022. PMID: 36212359 Free PMC article.
-
Regulation of Microalgal Photosynthetic Electron Transfer.Plants (Basel). 2024 Jul 29;13(15):2103. doi: 10.3390/plants13152103. Plants (Basel). 2024. PMID: 39124221 Free PMC article. Review.
References
-
- Allorent G, Guglielmino E, Giustini C, Courtois F. 2018. Generation of mutants of nuclear‐encoded plastid proteins using CRISPR/Cas9 in the diatom Phaeodactylum tricornutum . Methods in Molecular Biology 1829: 367–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

