The Use of Sustained Release Intravitreal Steroid Implants in Non-Infectious Uveitis Affecting the Posterior Segment of the Eye
- PMID: 35092605
- PMCID: PMC8800436
- DOI: 10.1007/s40123-022-00456-4
The Use of Sustained Release Intravitreal Steroid Implants in Non-Infectious Uveitis Affecting the Posterior Segment of the Eye
Abstract
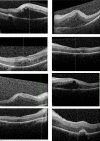
The treatment of non-infectious uveitis affecting the posterior segment of the eye has been revolutionized by the development of sustained-release corticosteroid implants over the past decade. Their use is now supported by healthcare systems that have licensed and commissioned them on the basis of the high-quality randomised controlled trials that formed part of their development and which have informed clinicians as to their benefits and risks. In particular, they have provided an alternative mode of treatment for patients who do not wish to be systemically immunosuppressed, or in whom such immunosuppression is less desirable, such as those with unilateral disease or those with concurrent illnesses such as diabetes mellitus, renal disease or osteoporosis that are negatively impacted by systemic corticosteroids or other immunosuppressive agents. In this article, we review the evidence for the use of the major licensed corticosteroid implants and assess the advantages and disadvantages of each.
Keywords: Corticosteroids; Iluvien; Local therapy; Ozurdex; Uveitis.
© 2022. The Author(s).
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

