Adenovirus-based vaccines-a platform for pandemic preparedness against emerging viral pathogens
- PMID: 35092844
- PMCID: PMC8801892
- DOI: 10.1016/j.ymthe.2022.01.034
Adenovirus-based vaccines-a platform for pandemic preparedness against emerging viral pathogens
Abstract
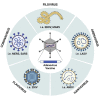
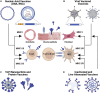
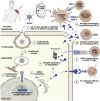
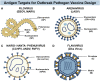
Zoonotic viruses continually pose a pandemic threat. Infection of humans with viruses for which we typically have little or no prior immunity can result in epidemics with high morbidity and mortality. These epidemics can have public health and economic impact and can exacerbate civil unrest or political instability. Changes in human behavior in the past few decades-increased global travel, farming intensification, the exotic animal trade, and the impact of global warming on animal migratory patterns, habitats, and ecosystems-contribute to the increased frequency of cross-species transmission events. Investing in the pre-clinical advancement of vaccine candidates against diverse emerging viral threats is crucial for pandemic preparedness. Replication-defective adenoviral (Ad) vectors have demonstrated their utility as an outbreak-responsive vaccine platform during the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Ad vectors are easy to engineer; are amenable to rapid, inexpensive manufacturing; are relatively safe and immunogenic in humans; and, importantly, do not require specialized cold-chain storage, making them an ideal platform for equitable global distribution or stockpiling. In this review, we discuss the progress in applying Ad-based vaccines against emerging viruses and summarize their global safety profile, as reflected by their widespread geographic use during the SARS-CoV-2 pandemic.
Keywords: adenovirus; emerging; outbreak; pandemic; pathogen; vaccine; vector; virus.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.C. declares no competing interests. E.J.K. is a member of the EMA vaccine advisory panel and was president of the user supervisory panel of TransVac II. D.M.S. is a paid consultant of Merck and a founder, officer, and shareholder of AdCure Bio, which develops adenovirus technologies for therapeutic use.
Figures
Similar articles
-
Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses.Front Immunol. 2021 Jan 29;11:607333. doi: 10.3389/fimmu.2020.607333. eCollection 2020. Front Immunol. 2021. PMID: 33633727 Free PMC article. Review.
-
Protective Efficacy of Rhesus Adenovirus COVID-19 Vaccines against Mouse-Adapted SARS-CoV-2.J Virol. 2021 Nov 9;95(23):e0097421. doi: 10.1128/JVI.00974-21. Epub 2021 Sep 15. J Virol. 2021. PMID: 34523968 Free PMC article.
-
Intranasal delivery of an adenovirus-vector vaccine co-expressing a modified spike protein and a genetic adjuvant confers lasting mucosal immunity against SARS-CoV-2.Antiviral Res. 2023 Aug;216:105656. doi: 10.1016/j.antiviral.2023.105656. Epub 2023 Jun 14. Antiviral Res. 2023. PMID: 37327877 Free PMC article.
-
Adenoviral Vector DNA- and SARS-CoV-2 mRNA-Based Covid-19 Vaccines: Possible Integration into the Human Genome - Are Adenoviral Genes Expressed in Vector-based Vaccines?Virus Res. 2021 Sep;302:198466. doi: 10.1016/j.virusres.2021.198466. Epub 2021 Jun 1. Virus Res. 2021. PMID: 34087261 Free PMC article. Review.
-
[Research and application of the SARS-CoV-2 vaccine based on adenovirus vector technology platform].Zhonghua Yu Fang Yi Xue Za Zhi. 2023 Jul 6;57(7):1082-1095. doi: 10.3760/cma.j.cn112150-20230419-00309. Zhonghua Yu Fang Yi Xue Za Zhi. 2023. PMID: 37198717 Review. Chinese.
Cited by
-
Optimization of Culture Media and Feeding Strategy for High Titer Production of an Adenoviral Vector in HEK 293 Fed-Batch Culture.Vaccines (Basel). 2024 May 10;12(5):524. doi: 10.3390/vaccines12050524. Vaccines (Basel). 2024. PMID: 38793775 Free PMC article.
-
An adenoviral vector encoding an inflammation-inducible antagonist, HMGB1 Box A, as a novel therapeutic approach to inflammatory diseases.mBio. 2025 Feb 5;16(2):e0338724. doi: 10.1128/mbio.03387-24. Epub 2024 Dec 19. mBio. 2025. PMID: 39699172 Free PMC article.
-
HBV Vaccines: Advances and Development.Vaccines (Basel). 2023 Dec 18;11(12):1862. doi: 10.3390/vaccines11121862. Vaccines (Basel). 2023. PMID: 38140265 Free PMC article. Review.
-
The Pseudotyped Replication-Deficient VSV with Spike from PEDV Induces Neutralizing Antibody Against PEDV.Vaccines (Basel). 2025 Feb 24;13(3):223. doi: 10.3390/vaccines13030223. Vaccines (Basel). 2025. PMID: 40266086 Free PMC article.
-
Adenoviral Vectors: Potential as Anti-HBV Vaccines and Therapeutics.Genes (Basel). 2022 Oct 25;13(11):1941. doi: 10.3390/genes13111941. Genes (Basel). 2022. PMID: 36360178 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

