Association of cellular HIV-1 DNA and virological success of antiretroviral treatment in HIV-infected sub-Saharan African adults
- PMID: 35093007
- PMCID: PMC8800335
- DOI: 10.1186/s12879-022-07082-2
Association of cellular HIV-1 DNA and virological success of antiretroviral treatment in HIV-infected sub-Saharan African adults
Abstract
Background: HIV-1 DNA persists in infected cells, forming viral reservoirs. Pre-antiretroviral treatment (ART) HIV-1 DNA load was reported to predict ART success in European severely immunocompromised patients. The aim of this study was to determine whether HIV-1 DNA levels are associated with virological success in less severely immunocompromised patients who receive early ART in sub-Saharan Africa.
Methods: The association between pre-ART HIV-1 DNA and the virological response after 30 months on ART was studied in multivariate logistic regression in patients randomised to immediate ART groups in the Temprano trial, which assessed the benefits of early ART in HIV-infected adults in Côte d'Ivoire. HIV-1 DNA was quantified in peripheral blood mononuclear cell (PBMC) using real-time PCR.
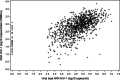
Results: HIV-1 DNA levels were measured in 1013 patients. Their medians [IQR] of pre-ART CD4 count, HIV-1 RNA and HIV-1 DNA levels were 465 [379-578]/mm3, 4.7 [4.0-5.3] log10 copies/ml and 2.9 [2.5-3.2] log10 copies/million PBMC, respectively. Pre-ART HIV-1 DNA was significantly correlated with pre-ART HIV-1 RNA (R = 0.59, p < 0.0001). In multivariate analysis, HIV-1 DNA < 3 log10 copies/million PBMC was significantly associated with virological success at M30 after adjustment for other key variables (ART regimen, IPT, sex, age, WHO clinical stage, CD4 and HIV-1 RNA; aOR 1.57; 95% CI 1.08-2.30; p = 0.02).
Conclusion: Low HIV-1 DNA was statistically associated with virological success in this population of sub-Saharan African adults who started treatment with a median pre-ART CD4 count at 465/mm3. HIV-1 DNA could become a useful tool for guiding some therapeutic decisions in the test-and-treat era. Trial registration TEMPRANO ANRS 12136 ClinicalTrials.gov, number NCT00495651, date of registration 03/07/2007.
Keywords: Africa; HIV-1 DNA; Test and treat; Therapeutic success.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, et al. a trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–22. - PubMed
-
- World Health Organization, World Health Organization, Department of HIV/AIDS. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. [Internet]. 2015 [cited 2019 Sep 4]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK327115/. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials