Impact of intra-partum azithromycin on carriage of group A streptococcus in the Gambia: a posthoc analysis of a double-blind randomized placebo-controlled trial
- PMID: 35093029
- PMCID: PMC8800276
- DOI: 10.1186/s12879-022-07080-4
Impact of intra-partum azithromycin on carriage of group A streptococcus in the Gambia: a posthoc analysis of a double-blind randomized placebo-controlled trial
Abstract
Background: Group A Streptococcus (GAS) is a major human pathogen and an important cause of maternal and neonatal sepsis. Asymptomatic bacterial colonization is considered a necessary step towards sepsis. Intra-partum azithromycin may reduce GAS carriage.
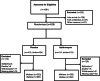
Methods: A posthoc analysis of a double-blind, placebo-controlled randomized-trial was performed to determine the impact of 2 g oral dose of intra-partum azithromycin on maternal and neonatal GAS carriage and antibiotic resistance. Following screening, 829 mothers were randomized who delivered 843 babies. GAS was determined by obtaining samples from the maternal and newborn nasopharynx, maternal vaginal tract and breastmilk. Whole Genome Sequencing (WGS) of GAS isolates was performed using the Illumina Miseq platform.
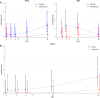
Results: GAS carriage was lower in the nasopharynx of both mothers and babies and breast milk among participants in the azithromycin arm. No differences in GAS carriage were found between groups in the vaginal tract. The occurrence of azithromycin-resistant GAS was similar in both arms, except for a higher prevalence in the vaginal tract among women in the azithromycin arm. WGS revealed all macrolide-resistant vaginal tract isolates from the azithromycin arm were Streptococcus dysgalactiae subspecies equisimilis expressing Lancefield group A carbohydrate (SDSE(A)) harbouring macrolide resistant genes msr(D) and mef(A). Ten of the 45 GAS isolates (22.2%) were SDSE(A).
Conclusions: Oral intra-partum azithromycin reduced GAS carriage among Gambian mothers and neonates however carriage in the maternal vaginal tract was not affected by the intervention due to azithromycin resistant SDSE(A). SDSE(A) resistance must be closely monitored to fully assess the public health impact of intrapartum azithromycin on GAS. Trial registration ClinicalTrials.gov Identifier NCT01800942.
Keywords: Azithromycin; Bacterial carriage; Group A streptococcus; Streptococcus dysgalactiae subspecies equisimilis; Sub-Saharan Africa.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures

References
-
- WHO. Maternal Sepsis. 2018 (cited 2018 10/02/2018). https://www.who.int/reproductivehealth/maternal-sepsis/en/.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- MR/J010391/1/MRC UK and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement
- HEFCE/ODA (155123)/The University of Sheffield
- Wellcome Trust Intermediate Clinical Fellowship (110058/Z/15/Z)/WT_/Wellcome Trust/United Kingdom
- Royal Society & Wellcome Trust Sir Henry Dale Fellow (208765/Z/17/Z)/Royal Society & Wellcome Trust
- MR/R010161/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical

