Dynamic regulation and functions of mRNA m6A modification
- PMID: 35093087
- PMCID: PMC8800407
- DOI: 10.1186/s12935-022-02452-x
Dynamic regulation and functions of mRNA m6A modification
Abstract
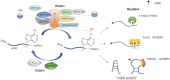
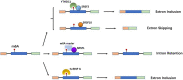
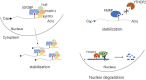
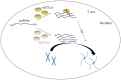
N6-Methyladenosine (m6A), the most abundant internal modification associated with eukaryotic mRNAs, has emerged as a dynamic regulatory mechanism controlling the expression of genes involved in many physiological activities by affecting various steps of mRNA metabolism, including splicing, export, translation, and stability. Here, we review the general role of m6A, highlighting recent advances related to the three major types enzymes that determine the level of m6A modification (i.e., writers, erasers, and readers) and the regulatory mechanism by which m6A influences multiple stages of RNA metabolism. This review clarifies the close connection and interaction between m6A modification and nuclear gene expression, and provides key background information for further studies of its roles in numerous physiological and pathophysiological processes. Among them, perhaps the most eye-catching process is tumorigenesis. Clarifying the molecular mechanism of tumorigenesis, development and metastasis in various tissues of the human body is conducive to curbing out-of-control cell activities from the root and providing a new strategy for human beings to defeat tumors.
Keywords: Gene expression; Mechanism; m6A; mRNA metabolism.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C, et al. Structural basis of N (6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534(7608):575–578. - PubMed
-
- Schapira M. Structural chemistry of human RNA methyltransferases. ACS Chem Biol. 2016;11(3):575–82. - PubMed
Publication types
Grants and funding
- 30801088/National Natural Science Foundation of China
- 202004a07020015/the Scientific Research Project of Anhui Province for the Prevention and Control of New Coronavirus Pneumonia
- 2019H214/the Scientific Research Project of Anhui Province for the Academic and technical leaders and candidates
- KJ2020ZD24/University Natural Science Research Project of Anhui Province
- KFJJ-2021-04/Open research fund of "Key Laboratory of anti-inflammatory and immune drugs of the Ministry of education" of Anhui Medical University
LinkOut - more resources
Full Text Sources

