The efficacy and safety of intra-articular injection of triamcinolone acetonide versus triamcinolone hexacetonide for treatment of juvenile idiopathic arthritis
- PMID: 35093116
- PMCID: PMC8801083
- DOI: 10.1186/s12969-022-00666-x
The efficacy and safety of intra-articular injection of triamcinolone acetonide versus triamcinolone hexacetonide for treatment of juvenile idiopathic arthritis
Abstract
Objectives: Juvenile idiopathic arthritis (JIA) is the most common childhood rheumatic disease. Intra-articular corticosteroids joint injection (IAJI), with triamcinolone hexacetonide (TH) or triamcinolone acetonide (TA), is an effective additional treatment for oligo and polyarticular JIA. Previous studies have shown the benefits of TH over TA; however, TA is still used in many pediatric rheumatology centers. Our unit has experience with both regimens, and therefore we aimed to compare the efficacy and safety of TA versus TH for JIA patients.
Methods: Chart review of JIA patients who were randomly (based on drug availability) treated with TA or TH IAJI during 2010-2019. Primary outcomes for efficacy were defined as full recovery from arthritis one month after IAJI and a relapse rate of arthritis 3 months after IAJI. Primary outcome for safety was defined as the occurrence of adverse events (AEs) during the follow up period after IAJI.
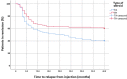
Results: Overall, 292 joints of 102 JIA patients were treated (138 TA/154 TH joints). Complete recovery after one month was documented in 107 (69.6%) of TA treated joints and 96 (69.5%) of TH treated joints (P = 0.232). However, rate of relapse after 3 months was significantly higher for TA treated joints (27 (20.1%) vs. 13 (8.8%), respectively, P < 0.01). No AEs were documented except minor scars at four joint injection sites.
Conclusion: The recovery from arthritis was similar (~ 70%) with both regimens, however relapse rate was more than double in TA as compared to TH injected joints. These findings are important due to a contemporary shortage of TH in the US market.
Keywords: Intra-articular corticosteroids; Juvenile idiopathic arthritis; Triamcinolone.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Ringold S. Horneff G - Oligoarticular juvenile IdiopathicArthritis. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, Fuhlbrigge R, Mellins E, editors. Textbook of pediatric rheumatology. Philadelphia, PA: Elsiever; 2020. pp. 241–249.
-
- Beukelman T, Patkar NM, Saag KG, Tolleson-Rinehart S, Cron RQ, DeWitt EM, et al. American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: initiation and safety monitoring of therapeutic agents for the treatment of arthritis and systemic features. Arthritis Care Res. 2011;63(4):465–482. doi: 10.1002/acr.20460. - DOI - PMC - PubMed
-
- Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non-systemic polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res. 2019;71(6):717–734. doi: 10.1002/acr.23870. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical