TRPV4 contributes to ER stress and inflammation: implications for Parkinson's disease
- PMID: 35093118
- PMCID: PMC8800324
- DOI: 10.1186/s12974-022-02382-5
TRPV4 contributes to ER stress and inflammation: implications for Parkinson's disease
Abstract
Background: Parkinson's disease (PD) is a progressive neurodegenerative disorder. Its molecular mechanism is still unclear, and pharmacological treatments are unsatisfactory. Transient receptor potential vanilloid 4 (TRPV4) is a nonselective Ca2+ channel. It has recently emerged as a critical risk factor in the pathophysiology of neuronal injuries and cerebral diseases. Our previous study reported that TRPV4 contributed to endoplasmic reticulum (ER) stress in the MPP+-induced cell model of PD. In the present study, we detected the role and the mechanism of TRPV4 in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice.
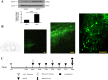
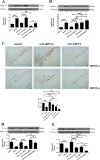
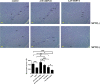
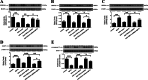
Methods: Intracerebral injection of an adeno-associated virus (AAV) into the substantia nigra (SN) of mice was used to knockdown or upregulate the expression of TRPV4 and intraperitoneal injection of MPTP. Rotarod and pole tests were used to evaluate the locomotor ability of mice. We used immunohistochemistry, Nissl staining and Western blot to detect the alterations in the number of tyrosine hydroxylase (TH)-positive neurons, Nissl-positive neurons, the levels of ER stress-associated molecules and proinflammatory cytokines in the SN.
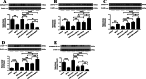
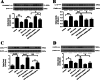
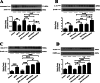
Results: The SN was transfected with AAV for 3 weeks and expressed the target protein with green fluorescence. Knockdown of TRPV4 via injection of a constructed AAV-TRPV4 shRNAi into the SN alleviated the movement deficits of PD mice. Upregulation of TRPV4 via injection of a constructed AAV-TRPV4 aggravated the above movement disorders. The expression of TRPV4 was upregulated in the SN of MPTP-treated mice. Injection of AAV-TRPV4 shRNAi into the SN rescued the number of TH-positive and Nissl-positive neurons in the SN decreased by MPTP, while injection of AAV-TRPV4 induced the opposite effect. Moreover, MPTP-decreased Sarco/endoplasmic reticulum Ca2+-ATPase 2 (SERCA2) and pro-cysteinyl aspartate specific proteinase-12 (procaspase-12), MPTP-increased Glucose-regulated protein 78 (GRP78), Glucose-regulated protein 94 (GRP94) and C/EBP homologous protein (CHOP) were inhibited by AAV-TRPV4 shRNAi infection, and enhanced by AAV-TRPV4. In the same way, MPTP-decreased procaspase-1, MPTP-increased Interleukin-18 (IL-18), Cyclooxgenase-2 (COX-2) and 5-Lipoxygenase (5-LOX) were inhibited by AAV-TRPV4 shRNAi, or further exacerbated by AAV-TRPV4.
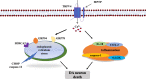
Conclusions: These results suggest that TRPV4 mediates ER stress and inflammation pathways, contributing to the loss of dopamine (DA) neurons in the SN and movement deficits in PD mice. Moreover, this study provides a new perspective on molecular targets and gene therapies for the treatment of PD in the future.
Keywords: ER stress; Inflammation; MPTP; Parkinson’s disease; SN; TRPV4.
© 2022. The Author(s).
Conflict of interest statement
All the authors declared no competing financial interests.
Figures

References
-
- Lai F, Jiang R, Xie W, et al. Intestinal pathology and gut microbiota alterations in a methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Neurochem Res. 2018;43(10):1986–1999. - PubMed
-
- Pupyshev AB, Tikhonova MA, Akopyan AA, et al. Therapeutic activation of autophagy by combined treatment with rapamycin and trehalose in a mouse MPTP-induced model of Parkinson's disease. Pharmacol Biochem Behav. 2019;177:1–11. - PubMed
-
- Zhu J, Dou S, Jiang Y, Chen J, et al. Apelin-13 protects dopaminergic neurons in MPTP-induced Parkinson's disease model mice through inhibiting endoplasmic reticulum stress and promoting autophagy. Brain Res. 2019;1715:203–212. - PubMed
-
- Li Y, Liu Z, Wang D, et al. Ucf-101 protects in vivoandin vitro models of PD against 6-hydroxydopamine toxicity by alleviating endoplasmic reticulum stress via the Wnt/β-catenin pathway. J Clin Neurosci. 2020;71:217–225. - PubMed
-
- Liu N, Liu J, Wen X, et al. TRPV4 contributes to ER stress: relation to apoptosis in the MPP+-induced cell model of Parkinson's disease. Life Sci. 2020;261:118461. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

