Pharmacological chaperones for the oxytocin receptor increase oxytocin responsiveness in myometrial cells
- PMID: 35093385
- PMCID: PMC8881472
- DOI: 10.1016/j.jbc.2022.101646
Pharmacological chaperones for the oxytocin receptor increase oxytocin responsiveness in myometrial cells
Abstract
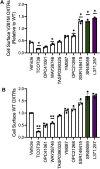
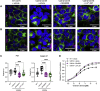
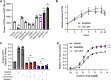
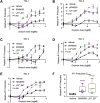
Oxytocin is a potent uterotonic agent administered to nearly all patients during childbirth in the United States. Inadequate oxytocin response can necessitate Cesarean delivery or lead to uterine atony and postpartum hemorrhage. Thus, it may be clinically useful to identify patients at risk for poor oxytocin response and develop strategies to sensitize the uterus to oxytocin. Previously, we showed that the V281M variant in the oxytocin receptor (OXTR) gene impairs OXTR trafficking to the cell surface, leading to a decreased oxytocin response in cells. Here, we sought to identify pharmacological chaperones that increased oxytocin response in cells expressing WT or V281M OXTR. We screened nine small-molecule agonists and antagonists of the oxytocin/vasopressin receptor family and identified two, SR49059 and L371,257, that restored both OXTR trafficking and oxytocin response in HEK293T cells transfected with V281M OXTR. In hTERT-immortalized human myometrial cells, which endogenously express WT OXTR, treatment with SR49059 and L371,257 increased the amount of OXTR on the cell surface by two- to fourfold. Furthermore, SR49059 and L371,257 increased the endogenous oxytocin response in hTERT-immortalized human myometrial cells by 35% and induced robust oxytocin responses in primary myometrial cells obtained from patients at the time of Cesarean section. If future studies demonstrate that these pharmacological chaperones or related compounds function similarly in vivo, we propose that they could potentially be used to enhance clinical response to oxytocin.
Keywords: myometrium; oxytocin; oxytocin receptor; pharmacological chaperones; pregnancy.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest M. M., Y. F., P. I. I., and S. K. E. have filed a provisional patent application (US 63/209,054) for compositions and methods for increasing cell surface OXTR. S. K. E., M. M., P. I. I., and Y. F. have patent #US 63/209,054 pending to Washington University in St. Louis.
Figures
References
-
- Martin J.A., Hamilton B.E., Osterman M.J.K., Driscoll A.K. Births: Final data for 2019. Natl. Vital Stat. Rep. 2021;70:1–51. - PubMed
-
- American College of Obstetrics and Gynecology Practice bulletin no. 183: Postpartum hemorrhage. Obstet. Gynecol. 2017;130:e168–e186. - PubMed
-
- Spong C.Y., Berghella V., Wenstrom K.D., Mercer B.M., Saade G.R. Preventing the first cesarean delivery: Summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, and American College of Obstetricians and Gynecologists workshop. In reply. Obstet. Gynecol. 2013;121:687. - PubMed
-
- Pergialiotis V., Bellos I., Antsaklis A., Papapanagiotou A., Loutradis D., Daskalakis G. Maternal and neonatal outcomes following a prolonged second stage of labor: A meta-analysis of observational studies. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:62–69. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

