Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2
- PMID: 35093538
- PMCID: PMC8801767
- DOI: 10.1016/j.ijantimicag.2022.106542
Remdesivir-ivermectin combination displays synergistic interaction with improved in vitro activity against SARS-CoV-2
Abstract
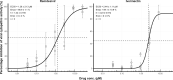
A key element for the prevention and management of coronavirus disease 2019 is the development of effective therapeutics. Drug combination strategies offer several advantages over monotherapies. They have the potential to achieve greater efficacy, to increase the therapeutic index of drugs and to reduce the emergence of drug resistance. We assessed the in vitro synergistic interaction between remdesivir and ivermectin, both approved by the US Food and Drug Administration, and demonstrated enhanced antiviral activity against severe acute respiratory syndrome coronavirus-2. Whilst the in vitro synergistic activity reported here does not support the clinical application of this combination treatment strategy due to insufficient exposure of ivermectin in vivo, the data do warrant further investigation. Efforts to define the mechanisms underpinning the observed synergistic action could lead to the development of novel treatment strategies.
Keywords: COVID-19; CPE; Combination therapy; Cytopathic activity; SARS-CoV-2; Synergy.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- World Health Organization . WHO; Geneva: 2020. WHO coronovirus disease (COVID-19) dashboard. Available at https://covid19whoint/
-
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

