Application experience of a rapid nucleic acid detection system for COVID-19
- PMID: 35093551
- PMCID: PMC8801965
- DOI: 10.1016/j.micinf.2022.104945
Application experience of a rapid nucleic acid detection system for COVID-19
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is raging worldwide. The COVID-19 outbreak caused severe threats to the life and health of all humans caused by SARS-CoV-2. Clinically, there is an urgent need for an in vitro diagnostic product to detect SARS-CoV-2 nucleic acid quickly. Under this background, commercial SARS-CoV-2 nucleic acid POCT products came into being. However, how to choose these products and how to use these products in a standardized way have brought new puzzles to clinical laboratories. This paper focuses on evaluating the performance of these commercial SARS-CoV-2 nucleic acid POCT products and helps the laboratory make the correct choice. At the same time, to standardize the use of this kind of product, this paper also puts forward corresponding suggestions from six elements of total quality management, namely, human, machine, material, method, environment, and measurement. In addition, this paper also puts forward some ideas on the future development direction of POCT products.
Keywords: COVID-19; Point-of-care testing; Quality control; SARS-CoV-2.
Copyright © 2022 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there are no conflicts of interest.
Figures
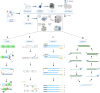
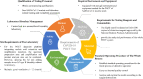
Similar articles
-
Recent advances and clinical application in point-of-care testing of SARS-CoV-2.J Med Virol. 2022 May;94(5):1866-1875. doi: 10.1002/jmv.27617. Epub 2022 Feb 5. J Med Virol. 2022. PMID: 35080017 Free PMC article. Review.
-
Laboratory-Generated DNA Can Cause Anomalous Pathogen Diagnostic Test Results.Microbiol Spectr. 2021 Oct 31;9(2):e0031321. doi: 10.1128/Spectrum.00313-21. Epub 2021 Sep 15. Microbiol Spectr. 2021. PMID: 34523989 Free PMC article.
-
Point of care molecular and antigen detection tests for COVID-19: current status and future prospects.Expert Rev Mol Diagn. 2022 Aug;22(8):797-809. doi: 10.1080/14737159.2022.2122712. Epub 2022 Sep 14. Expert Rev Mol Diagn. 2022. PMID: 36093682 Review.
-
An evaluation index system for regional mobile SARS-CoV-2 virus nucleic acid testing capacity in China: a modified Delphi consensus study.BMC Health Serv Res. 2022 Aug 24;22(1):1080. doi: 10.1186/s12913-022-08446-9. BMC Health Serv Res. 2022. PMID: 36002820 Free PMC article.
-
Detection of SARS-CoV-2 at the point of care.Bioanalysis. 2021 Aug;13(15):1213-1223. doi: 10.4155/bio-2021-0078. Epub 2021 Jul 22. Bioanalysis. 2021. PMID: 34289741 Free PMC article.
Cited by
-
Accurate Interpretation of SARS-CoV-2 Antigen Detection by Immunochromatography.Front Med (Lausanne). 2022 Jun 29;9:949554. doi: 10.3389/fmed.2022.949554. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35847813 Free PMC article. Review.
-
Evaluation of three rapid assays for detecting Mycoplasma pneumoniae in nasopharyngeal specimens.AMB Express. 2024 Dec 18;14(1):134. doi: 10.1186/s13568-024-01782-5. AMB Express. 2024. PMID: 39694968 Free PMC article.
-
Laboratory detection of SARS-CoV-2: A review of the current literature and future perspectives.Heliyon. 2022 Oct 3;8(10):e10858. doi: 10.1016/j.heliyon.2022.e10858. eCollection 2022 Oct. Heliyon. 2022. PMID: 36212015 Free PMC article. Review.
-
Assay methods based on proximity-enhanced reactions for detecting non-nucleic acid molecules.Front Bioeng Biotechnol. 2023 Jun 29;11:1188313. doi: 10.3389/fbioe.2023.1188313. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37456730 Free PMC article. Review.
-
Performance and application evaluation of SARS-CoV-2 antigen assay.J Med Virol. 2022 Aug;94(8):3548-3553. doi: 10.1002/jmv.27798. Epub 2022 Apr 30. J Med Virol. 2022. PMID: 35445404 Free PMC article. Review.
References
-
- Zachariah R., Harries A.D. The WHO clinical case definition for suspected cases of Ebola virus disease arriving at Ebola holding units: reason to worry? Lancet Infect Dis. 2015;15:989–990. - PubMed
-
- Li T.G., Wang M. Be alert to superposed effect of seasonal influenza while fighting against novel coronavirus pneumonia. Zhonghua Yufang Yixue Zazhi. 2020;54:E002. - PubMed
-
- Florkowski C., Don-Wauchope A., Gimenez N., Rodriguez-Capote K., Wils J., Zemlin A. Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM) - does it leverage any advantage in clinical decision making? Crit Rev Clin Lab Sci. 2017;54:471–494. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

