SMARCA4 biology in alveolar rhabdomyosarcoma
- PMID: 35094009
- PMCID: PMC9985831
- DOI: 10.1038/s41388-022-02205-0
SMARCA4 biology in alveolar rhabdomyosarcoma
Abstract
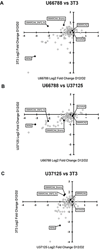
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and phenocopies a muscle precursor that fails to undergo terminal differentiation. The alveolar subtype (ARMS) has the poorest prognosis and represents the greatest unmet medical need for RMS. Emerging evidence supports the role of epigenetic dysregulation in RMS. Here we show that SMARCA4/BRG1, an ATP-dependent chromatin remodeling enzyme of the SWI/SNF complex, is prominently expressed in primary tumors from ARMS patients and cell cultures. Our validation studies for a CRISPR screen of 400 epigenetic targets identified SMARCA4 as a unique factor for long-term (but not short-term) tumor cell survival in ARMS. A SMARCA4/SMARCA2 protein degrader (ACBI-1) demonstrated similar long-term tumor cell dependence in vitro and in vivo. These results credential SMARCA4 as a tumor cell dependency factor and a therapeutic target in ARMS.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
CONFLICTS OF INTEREST
The Ciulli Laboratory receives or has received funding from Almirall, Amphista Therapeutics, Boehringer Ingelheim, Eisai, Nurix, and Ono Pharmaceuticals. A.C. is a scientific founder, shareholder, and consultant of Amphista Therapeutics, a company that is developing targeted protein degradation therapeutic platforms. C.K. is co-founder of Artisan Biopharma, a public benefit corporation pediatric cancer biopharma, as well as Tio Companies, and has received unrestricted grant support from Syndax Pharmaceuticals. C.K. also has research agreements with Roche/Genentech and Eli Lilly. All other authors declare no competing interests.
Figures




References
-
- Bharathy N, Suriyamurthy S, Rao VK, Ow JR, Lim HJ, Chakraborty P et al. P/CAF mediates PAX3-FOXO1-dependent oncogenesis in alveolar rhabdomyosarcoma. The Journal of pathology 2016; 240: 269–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

