Cancer-derived cholesterol sulfate is a key mediator to prevent tumor infiltration by effector T cells
- PMID: 35094065
- PMCID: PMC9020568
- DOI: 10.1093/intimm/dxac002
Cancer-derived cholesterol sulfate is a key mediator to prevent tumor infiltration by effector T cells
Abstract
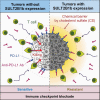
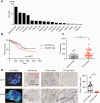
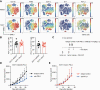
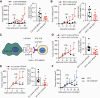
Effective tumor immunotherapy requires physical contact of T cells with cancer cells. However, tumors often constitute a specialized microenvironment that excludes T cells from the vicinity of cancer cells, and its underlying mechanisms are still poorly understood. DOCK2 is a Rac activator critical for migration and activation of lymphocytes. We herein show that cancer-derived cholesterol sulfate (CS), a lipid product of the sulfotransferase SULT2B1b, acts as a DOCK2 inhibitor and prevents tumor infiltration by effector T cells. Using clinical samples, we found that CS was abundantly produced in certain types of human cancers such as colon cancers. Functionally, CS-producing cancer cells exhibited resistance to cancer-specific T-cell transfer and immune checkpoint blockade. Although SULT2B1b is known to sulfate oxysterols and inactivate their tumor-promoting activity, the expression levels of cholesterol hydroxylases, which mediate oxysterol production, are low in SULT2B1b-expressing cancers. Therefore, SULT2B1b inhibition could be a therapeutic strategy to disrupt tumor immune evasion in oxysterol-non-producing cancers. Thus, our findings define a previously unknown mechanism for tumor immune evasion and provide a novel insight into the development of effective immunotherapies.
Keywords: DOCK2; SULT2B1b; immune evasion; tumor immunotherapy.
© The Author(s) 2022. Published by Oxford University Press on behalf of The Japanese Society for Immunology.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

