Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer
- PMID: 35094066
- PMCID: PMC10248839
- DOI: 10.1093/jnci/djac015
Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer
Erratum in
-
Correction to: Randomized trial of First-Line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-Mutated Non-Small cell lung cancer.J Natl Cancer Inst. 2023 Jun 8;115(6):773. doi: 10.1093/jnci/djad084. J Natl Cancer Inst. 2023. PMID: 37203415 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern to: Randomized Trial of First-Line Tyrosine Kinase Inhibitor With or Without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer.J Natl Cancer Inst. 2023 Jun 8;115(6):769. doi: 10.1093/jnci/djac065. J Natl Cancer Inst. 2023. PMID: 35362056 Free PMC article. No abstract available.
Abstract
Background: Adding radiotherapy (RT) to systemic therapy improves progression-free survival (PFS) and overall survival (OS) in oligometastatic non-small cell lung cancer (NSCLC). Whether these findings translate to epidermal growth factor receptor (EGFR)-mutated NSCLC remains unknown. The SINDAS trial (NCT02893332) evaluated first-line tyrosine kinase inhibitor (TKI) therapy for EGFR-mutated synchronous oligometastatic NSCLC and randomized to upfront RT vs no RT; we now report the prespecified interim analysis at 68% accrual.
Methods: Inclusion criteria were biopsy-proven EGFR-mutated adenocarcinoma (per amplification refractory mutation system or next generation sequencing), with synchronous (newly diagnosed, treatment naïve) oligometastatic (≤5 metastases; ≤2 lesions in any one organ) NSCLC without brain metastases. All patients received a first-generation TKI (gefitinib, erlotinib, or icotinib), and randomization was between no RT vs RT (25-40 Gy in 5 fractions depending on tumor size and location) to all metastases and the primary tumor/involved regional lymphatics. The primary endpoint (intention to treat) was PFS. Secondary endpoints included OS and toxicities. All statistical tests were 2-sided.
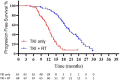
Results: A total of 133 patients (n = 65 TKI only, n = 68 TKI with RT) were enrolled (2016-2019). The median follow-up was 23.6 months. The respective median PFS was 12.5 months vs 20.2 months (P < .001), and the median OS was 17.4 months vs 25.5 months (P < .001) for TKI only vs TKI with RT. Treatment yielded no grade 5 events and a 6% rate of symptomatic grade 3-4 pneumonitis in the TKI with RT arm. Based on the efficacy results of this prespecified interim analysis, the ethics committee recommended premature cessation of this trial.
Conclusions: As compared with a first-line TKI alone, addition of upfront local therapy using RT statistically significantly improved PFS and OS for EGFR-mutated NSCLC.
© The Author(s) 2022. Published by Oxford University Press.
Figures

Comment in
-
Should We Target Oligometastatic EGFR-Mutated Non-Small Cell Lung Cancer With Radiotherapy Before Administering Targeted Systemic Therapy?J Natl Cancer Inst. 2023 Jun 8;115(6):605-607. doi: 10.1093/jnci/djac016. J Natl Cancer Inst. 2023. PMID: 35094086 Free PMC article. No abstract available.
References
-
- Mok TS, Wu YL, Thongprasert S, et al.Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. - PubMed
-
- Zhou C, Wu YL, Chen G, et al.Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

