Hospitalization Rates in Patients with Heart Failure and Reduced Ejection Fraction Initiating Sacubitril/Valsartan or Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers: A Retrospective Cohort Study
- PMID: 35094306
- PMCID: PMC8800553
- DOI: 10.1007/s40119-021-00252-4
Hospitalization Rates in Patients with Heart Failure and Reduced Ejection Fraction Initiating Sacubitril/Valsartan or Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers: A Retrospective Cohort Study
Abstract
Introduction: The angiotensin receptor neprilysin inhibitor (ARNI) sacubitril/valsartan (SAC/VAL) has shown benefit in patients with symptomatic heart failure (HF), including those naïve to renin-angiotensin-aldosterone system inhibitor (RAASi) therapy, and is considered the preferred RAASi for chronic HF. Real-world data on ARNI, specifically in RAASi-naïve patients, are limited. This study compared real-world outcomes of ARNI (SAC/VAL) vs. angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) therapy in RAASi-naïve patients with HF and reduced ejection fraction (HFrEF).
Methods: This retrospective cohort study included de-identified data on RAASi-naïve patients with HFrEF (left ventricular ejection fraction ≤ 40%) who had newly initiated SAC/VAL or ACEi/ARB between July 1, 2015, and March 31, 2019, from the Optum® Electronic Health Records database in the US. New SAC/VAL users were propensity score matched 1:2 with new ACEi/ARB users by pre-selected characteristics. One-year post-index rates of all-cause, HF, and cardiovascular hospitalizations and the composite of HF hospitalization or emergency room (ER) visits were measured using negative binomial regression. Time to first all-cause hospitalization, HF hospitalization, and composite of HF hospitalization or ER visits was measured using a subdistribution hazards model.
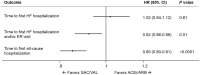
Results: The matched sample included 3059 new SAC/VAL and 6118 new ACEi/ARB users. Rates of all-cause hospitalization and composite of HF hospitalization or ER visits were significantly lower with SAC/VAL compared with ACEi/ARB (incidence rate ratio [95% confidence interval]: 0.87 [0.81-0.93] and 0.87 [0.81-0.94], respectively), whereas rates of HF hospitalizations and cardiovascular hospitalizations were similar (1.00 [0.91-1.11] and 0.94 [0.87-1.02], respectively). Time-to-event analyses also showed a similar trend.
Conclusions: In real-world clinical practice, RAASi-naïve patients with HFrEF initiating SAC/VAL were less likely to be hospitalized than those initiating ACEi/ARB, suggesting a potential for a reduced clinical and economic burden in these patients.
Keywords: Angiotensin receptor blocker; Angiotensin receptor neprilysin inhibitor; Angiotensin-converting enzyme inhibitor; Heart failure; Sacubitril/valsartan.
© 2022. The Author(s).
Figures



Similar articles
-
Real-world comparative effectiveness of sacubitril/valsartan versus RAS inhibition alone in patients with de novo heart failure.ESC Heart Fail. 2025 Jun;12(3):1682-1692. doi: 10.1002/ehf2.15183. Epub 2025 Jan 30. ESC Heart Fail. 2025. PMID: 39888167 Free PMC article.
-
Lower Hospitalization and Healthcare Costs With Sacubitril/Valsartan Versus Angiotensin-Converting Enzyme Inhibitor or Angiotensin-Receptor Blocker in a Retrospective Analysis of Patients With Heart Failure.J Am Heart Assoc. 2019 May 7;8(9):e011089. doi: 10.1161/JAHA.118.011089. J Am Heart Assoc. 2019. PMID: 31023122 Free PMC article.
-
Sacubitril/Valsartan Initiation Among Veterans Who Are Renin-Angiotensin-Aldosterone System Inhibitor Naïve With Heart Failure and Reduced Ejection Fraction.J Am Heart Assoc. 2021 Oct 19;10(20):e020474. doi: 10.1161/JAHA.120.020474. Epub 2021 Oct 6. J Am Heart Assoc. 2021. PMID: 34612065 Free PMC article.
-
Sacubitril/Valsartan in Asian Patients with Heart Failure with Reduced Ejection Fraction.Korean Circ J. 2019 Jun;49(6):469-484. doi: 10.4070/kcj.2019.0136. Korean Circ J. 2019. PMID: 31172710 Free PMC article. Review.
-
Neprilysin Inhibitors in Heart Failure: The Science, Mechanism of Action, Clinical Studies, and Unanswered Questions.JACC Basic Transl Sci. 2022 Sep 7;8(1):88-105. doi: 10.1016/j.jacbts.2022.05.010. eCollection 2023 Jan. JACC Basic Transl Sci. 2022. PMID: 36777165 Free PMC article. Review.
Cited by
-
Sacubitril/Valsartan in Heart Failure with Hypertension Patients: Real-World Experiences on Different Ages, Drug Doses, and Renal Functions.High Blood Press Cardiovasc Prev. 2023 Nov;30(6):561-572. doi: 10.1007/s40292-023-00606-0. Epub 2023 Nov 18. High Blood Press Cardiovasc Prev. 2023. PMID: 37979031
-
Icariin improves cardiac function and remodeling via the TGF-β1/Smad signaling pathway in rats following myocardial infarction.Eur J Med Res. 2023 Dec 19;28(1):607. doi: 10.1186/s40001-023-01588-4. Eur J Med Res. 2023. PMID: 38115154 Free PMC article.
-
Sacubitril/Valsartan in Heart Failure with Reduced Ejection Fraction: Real-World Experience from Italy (the REAL.IT Study).J Clin Med. 2023 Jan 16;12(2):699. doi: 10.3390/jcm12020699. J Clin Med. 2023. PMID: 36675628 Free PMC article.
-
Effect of sacubitril/valsartan on hospital readmissions in heart failure with reduced ejection fraction in Saudi Arabia: A multicenter retrospective cohort study.Medicine (Baltimore). 2024 Jul 26;103(30):e38960. doi: 10.1097/MD.0000000000038960. Medicine (Baltimore). 2024. PMID: 39058824 Free PMC article.
-
Real-world comparative effectiveness of sacubitril/valsartan versus RAS inhibition alone in patients with de novo heart failure.ESC Heart Fail. 2025 Jun;12(3):1682-1692. doi: 10.1002/ehf2.15183. Epub 2025 Jan 30. ESC Heart Fail. 2025. PMID: 39888167 Free PMC article.
References
-
- Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020;5:15. doi: 10.21037/amj.2020.03.03. - DOI
-
- Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169–1186. doi: 10.1002/ejhf.1531. - DOI - PubMed
-
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Cardiac Fail. 2017;23(8):628–651. doi: 10.1016/j.cardfail.2017.04.014. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

