Self-Induced Back-Action Actuated Nanopore Electrophoresis (SANE) Sensor for Label-Free Detection of Cancer Immunotherapy-Relevant Antibody-Ligand Interactions
- PMID: 35094337
- PMCID: PMC9207820
- DOI: 10.1007/978-1-0716-1811-0_20
Self-Induced Back-Action Actuated Nanopore Electrophoresis (SANE) Sensor for Label-Free Detection of Cancer Immunotherapy-Relevant Antibody-Ligand Interactions
Abstract
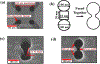
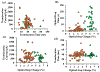
We fabricated a novel single molecule nanosensor by integrating a solid-state nanopore and a double nanohole nanoaperture. The nanosensor employs Self-Induced Back-Action (SIBA) for optical trapping and enables SIBA-Actuated Nanopore Electrophoresis (SANE) for concurrent acquisition of bimodal optical and electrical signatures of molecular interactions. This work describes how to fabricate and use the SANE sensor to quantify antibody-ligand interactions. We describe how to analyze the bimodal optical-electrical data to improve upon the discrimination of antibody and ligand versus bound complex compared to electrical measurements alone. Example results for specific interaction detection are described for T-cell receptor-like antibodies (TCRmAbs) engineered to target peptide-presenting Major Histocompatibility Complex (pMHC) ligands, representing a model of target ligands presented on the surface of cancer cells. We also describe how to analyze the bimodal optical-electrical data to discriminate between specific and non-specific interactions between antibodies and ligands. Example results for non-specific interactions are shown for cancer-irrelevant TCRmAbs targeting the same pMHCs, as a control. These example results demonstrate the utility of the SANE sensor as a potential screening tool for ligand targets in cancer immunotherapy, though we believe that its potential uses are much broader.
Keywords: Antibody-ligand interactions; Dual modality nanosensing; Dual nanoholes; Nanopore translocations; Peptide major histocompatibility complexes (pMHCs); Plasmonic optical trapping; Solid-state nanopores; TCR-like monoclonal antibodies.
© 2022. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
Figures




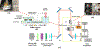





References
-
- Spitzberg JD, Zrehen A, van Kooten X F and Meller A 2019. Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection Advanced Materials 1900422 - PubMed
-
- Todd J, Freese B, Lu A, Held D, Morey J, Livingston R and Goix P 2007. Ultrasensitive flow-based immunoassays using single-molecule counting Clinical chemistry 53 1990–5 - PubMed
-
- Shim J-u, Ranasinghe RT, Smith CA, Ibrahim SM, Hollfelder F, Huck WT, Klenerman D and Abell C 2013. Ultrarapid generation of femtoliter microfluidic droplets for single-molecule-counting immunoassays Acs Nano 7 5955–64 - PubMed
-
- Hinterdorfer P and Dufrêne YF 2006. Detection and localization of single molecular recognition events using atomic force microscopy Nature methods 3 347. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

