Characterization of eosinophilic esophagitis variants by clinical, histological, and molecular analyses: A cross-sectional multi-center study
- PMID: 35094416
- PMCID: PMC9545458
- DOI: 10.1111/all.15233
Characterization of eosinophilic esophagitis variants by clinical, histological, and molecular analyses: A cross-sectional multi-center study
Abstract
Objective: Physicians are increasingly confronted with patients presenting with symptoms of esophageal dysfunction resembling eosinophilic esophagitis (EoE), but absence of significant esophageal eosinophilia. The purpose of this study was to characterize and classify this group of EoE variants.
Design: Patients from six EoE-centers with symptoms of esophageal dysfunction, but peak eosinophil counts of <60/mm2 (<15/hpf) in esophageal biopsies and absence of gastro-esophageal reflux disease (GERD) were included. Clinical, endoscopic, (immuno)-histological, and molecular features were determined and compared with EoE, GERD, and healthy controls.
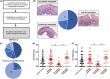
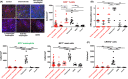
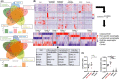
Results: We included 69 patients with EoE variants. Endoscopic abnormalities were found in 53.6%. We identified three histological subtypes: EoE-like esophagitis (36/69, 52.2%), lymphocytic esophagitis (14/69, 20.3%), and non-specific esophagitis (19/69, 27.5%). Immunohistochemistry revealed-in contrast to EoE-no significant increase in inflammatory cell infiltrates compared with GERD and healthy controls, except for lymphocytes in lymphocytic esophagitis. EoE-typical Th2-response was absent in all EoE variants. However, considerable structural changes were detected based on histology and protein expression. Using next generation mRNA sequencing, we found the three EoE variants to have distinct molecular fingerprints partially sharing pronounced traits of EoE. Hierarchical sample clustering of RNA sequencing data confirmed the presence of an EoE-like (characterized by eotaxin-3 expression), non-specific, and lymphocytic variant cluster (characterized by CD3 cells and TSLP expression).
Conclusion: All EoE variants are clinically and histologically active conditions despite the absence of esophageal eosinophilia. EoE variants appear to be part of a disease spectrum, where classical EoE represents the most common and apparent phenotype.
Keywords: dysphagia; eosinophilic esophagitis; esophageal eosinophilia; lymphocytic esophagitis; next generation rna sequencing.
© 2022 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
TG has consulting contracts with Sanofi‐Regeneron and Falk Pharma GmbH, received travel grants from Falk Pharma GmbH and Vifor, and an unrestricted research grant from Novartis. AS has consulting contracts with Actelion, Celgene‐Receptos, Falk Pharma GmbH, Roche‐Genentech, GSK, Novartis, Nutricia, and Sanofi‐Regeneron. ES is a consultant for Celgene Corp., Regeneron Pharmaceuticals Inc., and Novartis. AMS is a consultant for Falk Pharma GmbH, Adare Pharmaceuticals Inc, Celgene‐Receptos, and Sanofi‐Regeneron. MHC is a consultant for Astra Zeneca, Allakos, Arena, Celgene, Esocap, GlaxoSmithKline, Regeneron and Shire, and has received research funds from Regeneron and Shire. LB has received consulting fees and/or speaker fees from Falk Pharma GmbH, Esocap AG, Sanofi‐Aventis AG, and Calypso Biotech SA. GTF is a consultant for Takeda and Arena and has received research support from Holoclara. MC received research support from hire, Regeneron and Allakos, consulting fees from Shire, Regeneron, Allakos, Adare and Nutricia, and lecture honoraria from Nutricia, Medscape, and the Annenberg Center for Health Sciences at Eisenhower. IH has received consulting fees from Receptos, Regeneron, Shire, and Roche. ESD has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, Shire; consulting fees from Adare, Aimmune, Alivio, Allakos, AstraZeneca, Banner, Biorasi, Calypso, Celgene/Receptos, Enumeral, EsoCap, Gossamer Bio, GSK, Regeneron, Robarts, Salix, Shire; and educational grants from Allakos, Banner, Holoclara. HUS is a consultant for AstraZeneca, GlaxoSmithKline, and Esocap. The other authors have no competing interests to declare. No company representative was involved in conception, writing, or financing of this study.
Figures


References
-
- Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3‐20. - PubMed
-
- Straumann A, Conus S, Grzonka P, et al. Anti‐interleukin‐5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo‐controlled, double‐blind trial. Gut. 2010;59(1):21‐30. - PubMed
-
- Assa'ad AH, Gupta SK, Collins MH, et al. An antibody against IL‐5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141(5):1593‐1604. - PubMed
-
- Spergel JM, Rothenberg ME, Collins MH, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double‐blind, randomized, placebo‐controlled trial. J Allergy Clin Immunol. 2012;129(2):pp. 456‐463, 63. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

