Metabolomic analysis reveals potential biomarkers and the underlying pathogenesis involved in Mycoplasma pneumoniae pneumonia
- PMID: 35094669
- PMCID: PMC8865114
- DOI: 10.1080/22221751.2022.2036582
Metabolomic analysis reveals potential biomarkers and the underlying pathogenesis involved in Mycoplasma pneumoniae pneumonia
Abstract
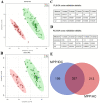
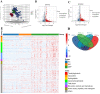
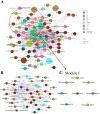
Although previous studies have reported the use of metabolomics for infectious diseases, little is known about the potential function of plasma metabolites in children infected with Mycoplasma pneumoniae (MP). Here, a combination of liquid chromatography-quadrupole time-of-flight mass spectrometry and random forest-based classification model was used to provide a broader range of applications in MP diagnosis. In the training cohort, plasma from 63 MP pneumonia children (MPPs), 37 healthy controls (HC) and 29 infectious disease controls (IDC) was collected. After multivariate analyses, 357 metabolites were identified to be differentially expressed among MPP, HC and IDC groups, and 3 metabolites (568.5661, 459.3493 and 411.3208) had high diagnostic values. In an independent cohort with 57 blinded subjects, samples were successfully classified into different groups, demonstrating the reliability of these biomarkers for distinguishing MPPs from controls. A metabolomic signature analysis identified major classes of glycerophospholipids, sphingolipids and fatty acyls were increased in MPPs. These markedly altered metabolites are mainly involved in glycerophospholipid and sphingolipid metabolism. As the ubiquitous building blocks of eukaryotic cell membranes, dysregulated lipid metabolism indicates damage of the cellular membrane and the activation of immunity in MPPs. Moreover, lipid metabolites, differentially expressed between severe and mild MPPs, were correlated with the markers of extrapulmonary complications, suggesting that they may be involved in MPP disease severity. These findings may offer new insights into biomarker selection and the pathogenesis of MPP in children.
Keywords: Mycoplasma pneumoniae pneumonia; metabolomics; children; diagnosis; pathogenesis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Lee KL, et al. . Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010–2019. J Formos Med Assoc. 2021;120(1 Pt 1):281–291. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
