Indium(II) Chloride as a Precursor in the Synthesis of Ternary (Ag-In-S) and Quaternary (Ag-In-Zn-S) Nanocrystals
- PMID: 35095188
- PMCID: PMC8794001
- DOI: 10.1021/acs.chemmater.1c03800
Indium(II) Chloride as a Precursor in the Synthesis of Ternary (Ag-In-S) and Quaternary (Ag-In-Zn-S) Nanocrystals
Abstract
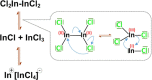
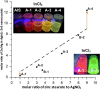
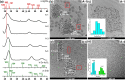
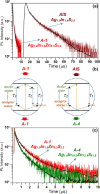
A new indium precursor, namely, indium(II) chloride, was tested as a precursor in the synthesis of ternary Ag-In-S and quaternary Ag-In-Zn-S nanocrystals. This new precursor, being in fact a dimer of Cl2In-InCl2 chemical structure, is significantly more reactive than InCl3, typically used in the preparation of these types of nanocrystals. This was evidenced by carrying out comparative syntheses under the same reaction conditions using these two indium precursors in combination with the same silver (AgNO3) and zinc (zinc stearate) precursors. In particular, the use of indium(II) chloride in combination with low concentrations of the zinc precursor yielded spherical-shaped (D = 3.7-6.2 nm) Ag-In-Zn-S nanocrystals, whereas for higher concentrations of this precursor, rodlike nanoparticles (L = 9-10 nm) were obtained. In all cases, the resulting nanocrystals were enriched in indium (In/Ag = 1.5-10.3). Enhanced indium precursor conversion and formation of anisotropic, longitudinal nanoparticles were closely related to the presence of thiocarboxylic acid type of ligands in the reaction mixture. These ligands were generated in situ and subsequently bound to surfacial In(III) cations in the growing nanocrystals. The use of the new precursor of enhanced reactivity facilitated precise tuning of the photoluminescence color of the resulting nanocrystals in the spectral range from ca. 730 to 530 nm with photoluminescence quantum yield (PLQY) varying from 20 to 40%. The fabricated Ag-In-S and Ag-In-Zn-S nanocrystals exhibited the longest, reported to date, photoluminescence lifetimes of ∼9.4 and ∼1.4 μs, respectively. It was also demonstrated for the first time that ternary (Ag-In-S) and quaternary (Ag-In-Zn-S) nanocrystals could be applied as efficient photocatalysts, active under visible light (green) illumination, in the reaction of aldehydes reduction to alcohols.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


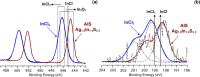
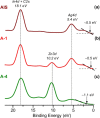

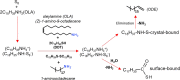

References
-
- Ye L.; Yong K.-T.; Liu L.; Roy I.; Hu R.; Zhu J.; Cai H.; Law W.-C.; Liu J.; Wang K.; Liu J.; Liu Y.; Hu Y.; Zhang X.; Swihart M. T.; Prasad P. N. A Pilot Study in Non-Human Primates Shows No Adverse Response to Interavenous Injection of Quantum Dots. Nat. Nanotechnol. 2012, 7, 453–458. 10.1038/nnano.2012.74. - DOI - PubMed
LinkOut - more resources
Full Text Sources
