Facile and Scalable Methodology for the Pyrrolo[2,1- f][1,2,4]triazine of Remdesivir
- PMID: 35095258
- PMCID: PMC8787819
- DOI: 10.1021/acs.oprd.1c00071
Facile and Scalable Methodology for the Pyrrolo[2,1- f][1,2,4]triazine of Remdesivir
Abstract
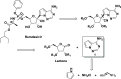
Pyrrolo[2,1-f][1,2,4]triazine (1) is an important regulatory starting material in the production of the antiviral drug remdesivir. Compound 1 was produced through a newly developed synthetic methodology utilizing simple building blocks such as pyrrole, chloramine, and formamidine acetate by examining the mechanistic pathway for the process optimization exercise. Triazine 1 was obtained in 55% overall yield in a two-vessel-operated process. This work describes the safety of the process, impurity profiles and control, and efforts toward the scale-up of triazine for the preparation of kilogram quantity.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Siegel D.; Hui H. C.; Doerffler E.; Clarke M. O.; Chun K.; Zhang L.; Neville S.; Carra E.; Lew W.; Ross B.; Wang Q.; Wolfe L.; Jordan R.; Soloveva V.; Knox J.; Perry J.; Perron M.; Stray K. M.; Barauskas O.; Feng J. Y.; Xu Y.; Lee G.; Rheingold A. L.; Ray A. S.; Bannister R.; Strickley R.; Swaminathan S.; Lee W. A.; Bavari S.; Cihlar T.; Lo M. K.; Warren T. K.; Mackman R. L. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60, 1648–1661. 10.1021/acs.jmedchem.6b01594. - DOI - PubMed
-
- Vieira T.; Stevens A. C.; Chtchemelinine A.; Gao D.; Badalov P.; Heumann L. Development of a Large-Scale Cyanation Process Using Continuous Flow Chemistry En Route to the Synthesis of Remdesivir. Org. Process Res. Dev. 2020, 24, 2113–2121. 10.1021/acs.oprd.0c00172. - DOI - PubMed
- Xue F.; Zhou X.; Zhou R.; Zhou X.; Xiao D.; Gu E.; Guo X.; Xiang J.; Wang K.; Yang L.; Zhong W.; Qin Y. Improvement of the C-glycosylation Step for the Synthesis of Remdesivir. Org. Process Res. Dev. 2020, 24, 1772–1777. 10.1021/acs.oprd.0c00310. - DOI - PubMed
- Wang M.; Zhang L.; Huo X.; Zhang Z.; Yuan Q.; Li P.; Chen J.; Zou Y.; Wu Z.; Zhang W. Catalytic Asymmetric Synthesis of the anti-COVID-19 Drug Remdesivir. Angew. Chem., Int. Ed. 2020, 59, 20814–20819. 10.1002/anie.202011527. - DOI - PubMed
- Bigley A. N.; Narindoshvili T.; Raushel F. M. A Chemoenzymatic Synthesis of the (RP)-Isomer of the Antiviral Prodrug Remdesivir. Biochemistry 2020, 59, 3038–3043. 10.1021/acs.biochem.0c00591. - DOI - PMC - PubMed
- von Keutz T.; Williams J. D.; Kappe C. O. Continuous Flow C-Glycosylation via Metal-Halogen Exchange: Process Understanding and Improvements toward Efficient Manufacturing of Remdesivir. Org. Process Res. Dev. 2020, 24, 2362–2368. 10.1021/acs.oprd.0c00370. - DOI
- De Savi C.; Hughes D. L.; Kvaerno L. Quest for a COVID-19 Cure by Repurposing Small-Molecule Drugs: Mechanism of Action, Clinical Development, Synthesis at Scale, and Outlook for Supply. Org. Process Res. Dev. 2020, 24, 940–976. 10.1021/acs.oprd.0c00233. - DOI - PubMed
-
- Paymode D. J.; Cardoso F. S. P.; Agrawal T.; Tomlin J. W.; Cook D. W.; Burns J. M.; Stringham R. W.; Sieber J. D.; Gupton B. F.; Snead D. R. Expanding Access to Remdesivir via an Improved Pyrrolotriazine Synthesis: Supply Centered Synthesis. Org. Lett. 2020, 22, 7656–7661. 10.1021/acs.orglett.0c02848. - DOI - PMC - PubMed
-
- Dixon J. A.; Phillips B.; Achebe F.; Kluender H. C. E.; Newcom J.; Parcella K.; Magnuson S.; Hong Z.; Zhang Z.; Liu Z.; Khire U.; Wang L.; Michels M.; Chandler B.; O’Connor S.. Substituted 4-amino-pyrrolotriazine derivatives useful for treating hyper-proliferative disorders and diseases associated with angiogenesis. U.S. Patent 8,143,393 B2, 2006.
- O’Connor S.; Dumas J.; Lee W.; Dixon J.; Cantin D.; Gunn D.; Burke J.; Phillips B.; Lowe D.; Shelekhin T.; Wang G.; Ma X.; Ying S.; McClure A.; Achebe F.; Lobell M.; Ehrgott F.; Iwuagwu C.; Parcella K.. Pyrrolo[2,1-f][1,2,4]triazin-4-ylamines IGF-1R kinase inhibitors for the treatment of cancer and other hyperproliferative diseases. U.S. Patent 8,431,695 B2, 2006.
-
- Achmatowicz M. M.; Thiel O. R.; Colyer J. T.; Hu J.; Elipe M. V. S.; Tomaskevitch J.; Tedrow J. S.; Larsen R. D. Hydrolysis of Phosphoryl Trichloride (POCl3): Characterization, in Situ Detection, and Safe Quenching of Energetic Metastable Intermediates. Org. Process Res. Dev. 2010, 14, 1490–1500. 10.1021/op1001484. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources