Impaired Glymphatic Function and Pulsation Alterations in a Mouse Model of Vascular Cognitive Impairment
- PMID: 35095472
- PMCID: PMC8793139
- DOI: 10.3389/fnagi.2021.788519
Impaired Glymphatic Function and Pulsation Alterations in a Mouse Model of Vascular Cognitive Impairment
Abstract
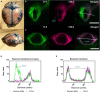
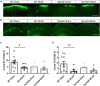
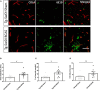
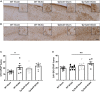
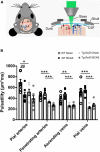
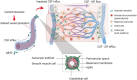
Large vessel disease and carotid stenosis are key mechanisms contributing to vascular cognitive impairment (VCI) and dementia. Our previous work, and that of others, using rodent models, demonstrated that bilateral common carotid stenosis (BCAS) leads to cognitive impairment via gradual deterioration of the neuro-glial-vascular unit and accumulation of amyloid-β (Aβ) protein. Since brain-wide drainage pathways (glymphatic) for waste clearance, including Aβ removal, have been implicated in the pathophysiology of VCI via glial mechanisms, we hypothesized that glymphatic function would be impaired in a BCAS model and exacerbated in the presence of Aβ. Male wild-type and Tg-SwDI (model of microvascular amyloid) mice were subjected to BCAS or sham surgery which led to a reduction in cerebral perfusion and impaired spatial learning acquisition and cognitive flexibility. After 3 months survival, glymphatic function was evaluated by cerebrospinal fluid (CSF) fluorescent tracer influx. We demonstrated that BCAS caused a marked regional reduction of CSF tracer influx in the dorsolateral cortex and CA1-DG molecular layer. In parallel to these changes increased reactive astrogliosis was observed post-BCAS. To further investigate the mechanisms that may lead to these changes, we measured the pulsation of cortical vessels. BCAS impaired vascular pulsation in pial arteries in WT and Tg-SwDI mice. Our findings show that BCAS influences VCI and that this is paralleled by impaired glymphatic drainage and reduced vascular pulsation. We propose that these additional targets need to be considered when treating VCI.
Keywords: amyloid-β (Aβ); carotid stenosis; cerebral amyloid angiopathy (CAA); glymphatic function; vascular cognitive impairment; vascular pulsation.
Copyright © 2022 Li, Kitamura, Beverley, Koudelka, Duncombe, Lennen, Jansen, Marshall, Platt, Wiegand, Carare, Kalaria, Iliff and Horsburgh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Aribisala B. S., Wiseman S., Morris Z., Valdes-Hernandez M. C., Royle N. A., Maniega S. M., et al. (2014b). Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke 45 605–607. 10.1161/STROKEAHA.113.004059 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

