Targeting HSP90 Inhibits Proliferation and Induces Apoptosis Through AKT1/ERK Pathway in Lung Cancer
- PMID: 35095481
- PMCID: PMC8795737
- DOI: 10.3389/fphar.2021.724192
Targeting HSP90 Inhibits Proliferation and Induces Apoptosis Through AKT1/ERK Pathway in Lung Cancer
Abstract
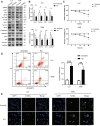
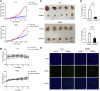
Lung cancer is one of the most common malignant cancers worldwide. Searching for specific cancer targets and developing efficient therapies with lower toxicity is urgently needed. HPS90 is a key chaperon protein that has multiple client proteins involved in the development of cancer. In this study, we investigated the transcriptional levels of HSP90 isoforms in cancerous and normal tissues of lung cancer patients in multiple datasets. The higher expression of HSP90AA1 in cancer tissues correlated with poorer overall survival was observed. The higher levels of transcription and expression of HSP90AA1 and the activity of AKT1/ERK pathways were confirmed in lung cancer patient tissues. In both human and mouse lung cancer cell lines, knocking down HSP90AA1 promoted cell apoptosis through the inhibition of the pro-survival effect of AKT1 by decreasing the phosphorylation of itself and its downstream factors of mTOR and BAD, as well as downregulating Mcl1, Bcl-xl, and Survivin. The knockdown also suppressed lung cancer cell proliferation by inhibiting ERK activation and downregulating CyclinD1 expression. The treatment of 17-DMAG, an HSP90 inhibitor, recaptured these effects in vitro and inhibited tumor cell growth, and induced apoptosis without obvious side effects in lung tumor xenograft mouse models. This study suggests that targeting HSP90 by 17-DMAG could be a potential therapy for the treatment of lung cancer.
Keywords: 17-DMAG; AKT1; ERK; Hsp90; lung cancer.
Copyright © 2022 Niu, Zhang, Li, Su, Pu, Zhao, Wei, Lian, Lu, Wang, Wazir, Gao, Song and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Brognard J., Clark A. S., Ni Y., Dennis P. A. (2001). Akt/protein Kinase B Is Constitutively Active in Non-small Cell Lung Cancer Cells and Promotes Cellular Survival and Resistance to Chemotherapy and Radiation. Cancer Res. 61 (10), 3986–3997. - PubMed
-
- Bucci M., Roviezzo F., Cicala C., Sessa W. C., Cirino G. (2000). Geldanamycin, an Inhibitor of Heat Shock Protein 90 (Hsp90) Mediated Signal Transduction Has Anti-inflammatory Effects and Interacts with Glucocorticoid Receptor In Vivo . Br. J. Pharmacol. 131 (1), 13–16. 10.1038/sj.bjp.0703549 - DOI - PMC - PubMed
-
- Chatterjee S., Bhattacharya S., Socinski M. A., Burns T. F. (2016). HSP90 Inhibitors in Lung Cancer: Promise Still Unfulfilled. Clin. Adv. Hematol. Oncol. 14 (5), 346–356. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

