Chronic Exposure to Cigarette Smoke Affects the Ileum and Colon of Guinea Pigs Differently. Relaxin (RLX-2, Serelaxin) Prevents Most Local Damage
- PMID: 35095510
- PMCID: PMC8793690
- DOI: 10.3389/fphar.2021.804623
Chronic Exposure to Cigarette Smoke Affects the Ileum and Colon of Guinea Pigs Differently. Relaxin (RLX-2, Serelaxin) Prevents Most Local Damage
Abstract
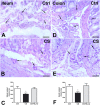
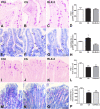
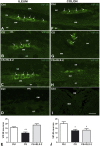
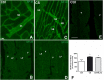
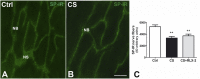
Cigarette smoking (CS) is the cause of several organ and apparatus diseases. The effects of smoke in the gut are partially known. Accumulating evidence has shown a relationship between smoking and inflammatory bowel disease, prompting us to investigate the mechanisms of action of smoking in animal models. Despite the role played by neuropeptides in gut inflammation, there are no reports on their role in animal models of smoking exposure. The hormone relaxin has shown anti-inflammatory properties in the intestine, and it might represent a putative therapy to prevent gut damage caused by smoking. Presently, we investigate the effects of chronic smoke exposure on inflammation, mucosal secretion, and vasoactive intestinal peptide (VIP) and substance P (SP) expressions in the ileum and colon of guinea pigs. We also verify the ability of relaxin to counter the smoke-induced effects. Smoke impacted plasma carbon monoxide (CO). In the ileum, it induced inflammatory infiltrates, fibrosis, and acidic mucin production; reduced the blood vessel area; decreased c-kit-positive mast cells and VIP-positive neurons; and increased the SP-positive nerve fibers. In the colon, it reduced the blood vessel area and the goblet cell area and decreased c-kit-positive mast cells, VIP-positive neurons, and SP-positive nerve fibers. Relaxin prevented most of the smoking-induced changes in the ileum, while it was less effective in the colon. This study shows the diverse sensitivity to CS between the ileum and the colon and demonstrates that both VIP and SP are affected by smoking. The efficacy of relaxin proposes this hormone as a potential anti-inflammatory therapeutic to counteract gut damage in humans affected by inflammatory bowel diseases.
Keywords: blood vessels; inflammation; mast cells; mucins; relaxin hormone; substance P; vasoactive intestinal peptide.
Copyright © 2022 Traini, Nistri, Calosi and Vannucchi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Protection from Cigarette Smoke-Induced Lung Dysfunction and Damage by H2 Relaxin (Serelaxin).J Pharmacol Exp Ther. 2016 Jun;357(3):451-8. doi: 10.1124/jpet.116.232215. Epub 2016 Apr 5. J Pharmacol Exp Ther. 2016. PMID: 27048661
-
Protection from cigarette smoke-induced vascular injury by recombinant human relaxin-2 (serelaxin).J Cell Mol Med. 2016 May;20(5):891-902. doi: 10.1111/jcmm.12802. Epub 2016 Feb 24. J Cell Mol Med. 2016. PMID: 26915460 Free PMC article.
-
Translational research into the effects of cigarette smoke on inflammatory mediators and epithelial TRPV1 in Crohn's disease.PLoS One. 2020 Aug 6;15(8):e0236657. doi: 10.1371/journal.pone.0236657. eCollection 2020. PLoS One. 2020. PMID: 32760089 Free PMC article.
-
Serelaxin as a novel therapeutic opposing fibrosis and contraction in lung diseases.Pharmacol Ther. 2018 Jul;187:61-70. doi: 10.1016/j.pharmthera.2018.02.004. Epub 2018 Feb 12. Pharmacol Ther. 2018. PMID: 29447958 Review.
-
Role of Substance P in the Pathophysiology of Inflammatory Bowel Disease and Its Correlation With the Degree of Inflammation.Cureus. 2020 Oct 18;12(10):e11027. doi: 10.7759/cureus.11027. Cureus. 2020. PMID: 33214955 Free PMC article. Review.
Cited by
-
Chronic Exposure to Both Electronic and Conventional Cigarettes Alters Ileum and Colon Turnover, Immune Function, and Barrier Integrity in Mice.J Xenobiot. 2024 Jul 22;14(3):950-969. doi: 10.3390/jox14030053. J Xenobiot. 2024. PMID: 39051349 Free PMC article.
-
Homoplantaginin inhibits the progression of ulcerative colitis in mice by regulating the MMP9-RLN2 signaling axis.Front Med (Lausanne). 2025 Jun 2;12:1582066. doi: 10.3389/fmed.2025.1582066. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40529132 Free PMC article.
-
Cigarette smoke-induced dysbiosis: comparative analysis of lung and intestinal microbiomes in COPD mice and patients.Respir Res. 2024 May 10;25(1):204. doi: 10.1186/s12931-024-02836-9. Respir Res. 2024. PMID: 38730440 Free PMC article.
-
Otilonium Bromide Prevents Cholinergic Changes in the Distal Colon Induced by Chronic Water Avoidance Stress, a Rat Model of Irritable Bowel Syndrome.Int J Mol Sci. 2023 Apr 18;24(8):7440. doi: 10.3390/ijms24087440. Int J Mol Sci. 2023. PMID: 37108603 Free PMC article.
-
The Mechanism of "Treating Different Diseases with the Same Treatment" by Qiangji Jianpi Decoction in Ankylosing Spondylitis Combined with Inflammatory Bowel Disease: A Comprehensive Analysis of Multiple Methods.Gastroenterol Res Pract. 2024 May 21;2024:9709260. doi: 10.1155/2024/9709260. eCollection 2024. Gastroenterol Res Pract. 2024. PMID: 38808131 Free PMC article.
References
LinkOut - more resources
Full Text Sources

