Influence of High Hemoglobin-Oxygen Affinity on Humans During Hypoxia
- PMID: 35095551
- PMCID: PMC8795792
- DOI: 10.3389/fphys.2021.763933
Influence of High Hemoglobin-Oxygen Affinity on Humans During Hypoxia
Abstract
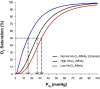
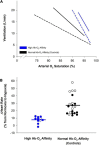
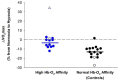
Humans elicit a robust series of physiological responses to maintain adequate oxygen delivery during hypoxia, including a transient reduction in hemoglobin-oxygen (Hb-O2) affinity. However, high Hb-O2 affinity has been identified as a beneficial adaptation in several species that have been exposed to high altitude for generations. The observed differences in Hb-O2 affinity between humans and species adapted to high altitude pose a central question: is higher or lower Hb-O2 affinity in humans more advantageous when O2 availability is limited? Humans with genetic mutations in hemoglobin structure resulting in high Hb-O2 affinity have shown attenuated cardiorespiratory adjustments during hypoxia both at rest and during exercise, providing unique insight into this central question. Therefore, the purpose of this review is to examine the influence of high Hb-O2 affinity during hypoxia through comparison of cardiovascular and respiratory adjustments elicited by humans with high Hb-O2 affinity compared to those with normal Hb-O2 affinity.
Keywords: VO2max (maximal oxygen uptake); altitude acclimatization; exercise; high affinity hemoglobin (Hb); high-altitude; oxygen transport.
Copyright © 2022 Webb, Dominelli, Baker, Klassen, Joyner, Senefeld and Wiggins.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Aste-Salazar H., Hurtado A. (1944). The affinity of hemoglobin for oxygen at sea level and at high altitudes. Am. J. Physiol. Legacy Content 142 733–743. 10.1152/ajplegacy.1944.142.5.733 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

