PFKP Activation Ameliorates Foot Process Fusion in Podocytes in Diabetic Kidney Disease
- PMID: 35095764
- PMCID: PMC8794994
- DOI: 10.3389/fendo.2021.797025
PFKP Activation Ameliorates Foot Process Fusion in Podocytes in Diabetic Kidney Disease
Abstract
Background: Glycolysis dysfunction is an important pathogenesis of podocyte injury in diabetic kidney disease (DKD). Foot process fusion of podocytes and increased albuminuria are markers of early DKD. Moreover, cytoskeletal remodeling has been found to be involved in the foot process fusion of podocytes. However, the connections between cytoskeletal remodeling and alterations of glycolysis in podocytes in DKD have not been clarified.
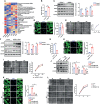
Methods: mRNA sequencing of glomeruli obtained from db/db and db/m mice with albuminuria was performed to analyze the expression profiling of genes in glucose metabolism. Expressions of phosphofructokinase platelet type (PFKP) in the glomeruli of DKD patients were detected. Clotrimazole (CTZ) was used to explore the renal effects of PFKP inhibition in diabetic mice. Using Pfkp siRNA or recombinant plasmid to manipulate PFKP expression, the effects of PFKP on high glucose (HG) induced podocyte damage were assessed in vitro. The levels of fructose-1,6-bisphosphate (FBP) were measured. Targeted metabolomics was performed to observe the alterations of the metabolites in glucose metabolism after HG stimulation. Furthermore, aldolase type b (Aldob) siRNA or recombinant plasmid were applied to evaluate the influence of FBP level alteration on podocytes. FBP was directly added to podocyte culture media. Db/db mice were treated with FBP to investigate its effects on their kidney.
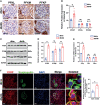
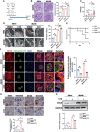
Results: mRNA sequencing showed that glycolysis enzyme genes were altered, characterized by upregulation of upstream genes (Hk1, and Pfkp) and down-regulation of downstream genes of glycolysis (Pkm, and Ldha). Moreover, the expression of PFKP was increased in glomeruli of DKD patients. The CTZ group presented more severe renal damage. In vitro, the Pfkp siRNA group and ALDOB overexpression group showed much more induced cytoskeletal remodeling in podocytes, while overexpression of PFKP and suppression of ALDOB in vitro rescued podocytes from cytoskeletal remodeling through regulation of FBP levels and inhibition of the RhoA/ROCK1 pathway. Furthermore, targeted metabolomics showed FBP level was significantly increased in HG group compared with the control group. Exogenous FBP addition reduced podocyte cytoskeletal remodeling and renal damage of db/db mice.
Conclusions: These findings provide evidence that PFKP may be a potential target for podocyte injury in DN and provide a rationale for applying podocyte glycolysis enhancing agents in patients with DKD.
Keywords: FBP; PFKP; cytoskeletal remodeling; diabetic kidney diseases; glycolysis; podocyte injury.
Copyright © 2022 Zhang, Liang, Luo, Hu, Yang, Hu, Chen, Zhu, Feng, Zhu, Chi and Ding.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Sirt6 ameliorates high glucose-induced podocyte cytoskeleton remodeling via the PI3K/AKT signaling pathway.Ren Fail. 2024 Dec;46(2):2410396. doi: 10.1080/0886022X.2024.2410396. Epub 2024 Oct 8. Ren Fail. 2024. PMID: 39378103 Free PMC article.
-
Compromised glycolysis contributes to foot process fusion of podocytes in diabetic kidney disease: Role of ornithine catabolism.Metabolism. 2022 Sep;134:155245. doi: 10.1016/j.metabol.2022.155245. Epub 2022 Jun 30. Metabolism. 2022. PMID: 35780908
-
Dihydroxyacetone phosphate accumulation leads to podocyte pyroptosis in diabetic kidney disease.J Cell Mol Med. 2024 Feb;28(3):e18073. doi: 10.1111/jcmm.18073. Epub 2023 Dec 8. J Cell Mol Med. 2024. PMID: 38063077 Free PMC article.
-
Signaling Pathways of Podocyte Injury in Diabetic Kidney Disease and the Effect of Sodium-Glucose Cotransporter 2 Inhibitors.Cells. 2022 Dec 3;11(23):3913. doi: 10.3390/cells11233913. Cells. 2022. PMID: 36497173 Free PMC article. Review.
-
Podocyte programmed cell death in diabetic kidney disease: Molecular mechanisms and therapeutic prospects.Biomed Pharmacother. 2024 Aug;177:117140. doi: 10.1016/j.biopha.2024.117140. Epub 2024 Jul 16. Biomed Pharmacother. 2024. PMID: 39018872 Review.
Cited by
-
Downregulating lncRNA MIAT attenuates apoptosis of podocytes exposed to high glucose.Acta Diabetol. 2024 Apr;61(4):451-460. doi: 10.1007/s00592-023-02213-w. Epub 2023 Dec 11. Acta Diabetol. 2024. PMID: 38072843
-
Irisin Ameliorates Renal Tubulointerstitial Fibrosis by Regulating the Smad4/β-Catenin Pathway in Diabetic Mice.Diabetes Metab Syndr Obes. 2023 Jun 2;16:1577-1593. doi: 10.2147/DMSO.S407734. eCollection 2023. Diabetes Metab Syndr Obes. 2023. PMID: 37292142 Free PMC article.
-
P16-positive senescent cells promote DKD by the dysregulation of glycolysis and mitochondrial metabolism.Cell Death Discov. 2025 Jul 30;11(1):355. doi: 10.1038/s41420-025-02650-2. Cell Death Discov. 2025. PMID: 40738954 Free PMC article.
-
Epigenetic modification in diabetic kidney disease.Front Endocrinol (Lausanne). 2023 Jun 30;14:1133970. doi: 10.3389/fendo.2023.1133970. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37455912 Free PMC article. Review.
-
Inhibition of PFKP in renal tubular epithelial cell restrains TGF-β induced glycolysis and renal fibrosis.Cell Death Dis. 2023 Dec 12;14(12):816. doi: 10.1038/s41419-023-06347-1. Cell Death Dis. 2023. PMID: 38086793 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

