Emerging Parasitic Protists: The Case of Perkinsea
- PMID: 35095782
- PMCID: PMC8792838
- DOI: 10.3389/fmicb.2021.735815
Emerging Parasitic Protists: The Case of Perkinsea
Abstract
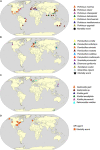
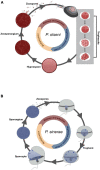
The last century has witnessed an increasing rate of new disease emergence across the world leading to permanent loss of biodiversity. Perkinsea is a microeukaryotic parasitic phylum composed of four main lineages of parasitic protists with broad host ranges. Some of them represent major ecological and economical threats because of their geographically invasive ability and pathogenicity (leading to mortality events). In marine environments, three lineages are currently described, the Parviluciferaceae, the Perkinsidae, and the Xcellidae, infecting, respectively, dinoflagellates, mollusks, and fish. In contrast, only one lineage is officially described in freshwater environments: the severe Perkinsea infectious agent infecting frog tadpoles. The advent of high-throughput sequencing methods, mainly based on 18S rRNA assays, showed that Perkinsea is far more diverse than the previously four described lineages especially in freshwater environments. Indeed, some lineages could be parasites of green microalgae, but a formal nature of the interaction needs to be explored. Hence, to date, most of the newly described aquatic clusters are only defined by their environmental sequences and are still not (yet) associated with any host. The unveiling of this microbial black box presents a multitude of research challenges to understand their ecological roles and ultimately to prevent their most negative impacts. This review summarizes the biological and ecological traits of Perkinsea-their diversity, life cycle, host preferences, pathogenicity, and highlights their diversity and ubiquity in association with a wide range of hosts.
Keywords: Parvilucifera; Perkinsus; X-cell parasite; broad host range parasite; emerging diseases; opportunistic parasite; severe Perkinsea infection.
Copyright © 2022 Itoïz, Metz, Derelle, Reñé, Garcés, Bass, Soudant and Chambouvet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Agosta S. J., Janz N., Brooks D. R. (2010). How specialists can be generalists: resolving the “parasite paradox” and implications for emerging infectious disease. Zoologia 27 151–162. 10.1590/S1984-46702010000200001 - DOI
-
- Alacid E., Reñé A., Gallisai R., Paloheimo A., Garcés E., Kremp A. (2020). Description of two new coexisting parasitoids of blooming dinoflagellates in the Baltic sea: Parvilucifera catillosa sp. nov. and Parvilucifera sp. (Perkinsea, Alveolata). Harmful Algae 100:101944. 10.1016/j.hal.2020.101944 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

