Neutrophil-Mediated Immunopathology and Matrix Metalloproteinases in Central Nervous System - Tuberculosis
- PMID: 35095865
- PMCID: PMC8789671
- DOI: 10.3389/fimmu.2021.788976
Neutrophil-Mediated Immunopathology and Matrix Metalloproteinases in Central Nervous System - Tuberculosis
Abstract
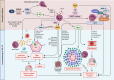
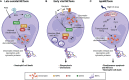
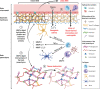
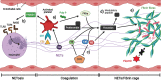
Tuberculosis (TB) remains one of the leading infectious killers in the world, infecting approximately a quarter of the world's population with the causative organism Mycobacterium tuberculosis (M. tb). Central nervous system tuberculosis (CNS-TB) is the most severe form of TB, with high mortality and residual neurological sequelae even with effective TB treatment. In CNS-TB, recruited neutrophils infiltrate into the brain to carry out its antimicrobial functions of degranulation, phagocytosis and NETosis. However, neutrophils also mediate inflammation, tissue destruction and immunopathology in the CNS. Neutrophils release key mediators including matrix metalloproteinase (MMPs) which degrade brain extracellular matrix (ECM), tumor necrosis factor (TNF)-α which may drive inflammation, reactive oxygen species (ROS) that drive cellular necrosis and neutrophil extracellular traps (NETs), interacting with platelets to form thrombi that may lead to ischemic stroke. Host-directed therapies (HDTs) targeting these key mediators are potentially exciting, but currently remain of unproven effectiveness. This article reviews the key role of neutrophils and neutrophil-derived mediators in driving CNS-TB immunopathology.
Keywords: central nervous system tuberculosis; host-directed therapy; matrix metalloproteinases; neutrophils; stroke; tuberculosis.
Copyright © 2022 Poh, Loh, Friedland and Ong.
Conflict of interest statement
CO received speaking fees from Qiagen outside this work. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Global Tuberculosis Report 2020. Geneva: World Health Organization. (2020). p. 232. Licence: CC BY-NC-SA 3.0 IGO
-
- Rom WN, Garay SM. Tuberculosis. 2 Ed. Philadelphia: Lippincott Williams & Wilkins; (2004).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

